Molekylære nanocarboner med mekaniske bindinger
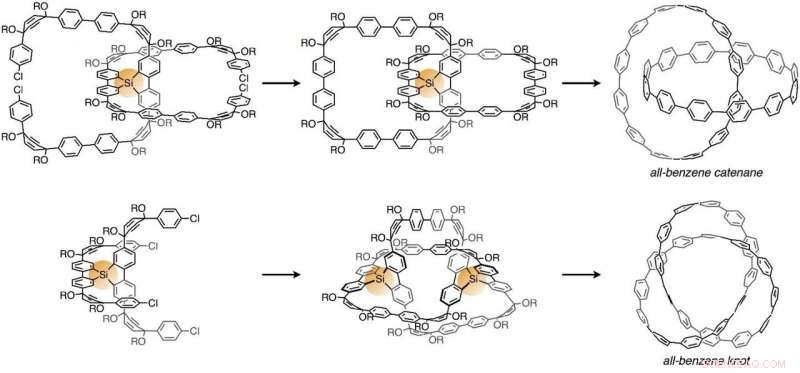
Syntesevej af en hel-benzen catenan (øverst) og knude (detaljer). Først, chloratomerne (Cl) dele blev reageret gennem en reaktion med nikkel for at danne carbon-carbon-bindinger. Efter det, siliciumatomerne blev fjernet ved anvendelse af fluor, og derefter blev de krympede dele (OR) fjernet ved en reaktion med natrium for at omdanne til benzenringe. Kredit:Nogoya University
Kulstofmaterialer med periodicitet i nanoskala, såsom grafen og kulstofnanorør, kaldet "nanocarboner, " forventes at blive lys, meget funktionelle næste generations materialer. Der har været krav om præcise syntesemetoder, der kun er rettet mod nanocarbonstrukturen med den ønskede egenskab, fordi deres elektroniske og mekaniske egenskaber er meget forskellige afhængigt af strukturen. Under sådanne omstændigheder, "molekylær nanocarbonvidenskab, "hvor organisk syntese bruges til præcist at syntetisere molekyler med partielle strukturer af nanocarboner, har på det seneste fået opmærksomhed og dermed er en masse forskningsprojekter på vej over hele verden.
Indtil nu, en masse molekyler med partielle strukturer af fullerener, grafen og kulstof nanorør (molekylære nanocarboner) er blevet syntetiseret. De har, imidlertid, relativt enklere strukturer set fra topologi. På den anden side, et stort antal nanocarboner med en kompleks topologi, såsom doughnutformede (torus) eller spoleformede, er blevet forudsagt ud fra teoretisk kemi, og derfor har forskere interesseret sig for sådanne nanocarboners ukendte egenskaber. Som det første trin i præcis syntese af sådanne nanocarboner, et hold ledet af Yasutomo Segawa, en gruppeleder af JST-ERATO-projektet, og Kenichiro Itami, direktøren for JST-ERATO-projektet og centerdirektøren for ITbM har foreslået "topologiske molekylære nanocarboner, " som er molekylære nanocarboner med en kompleks topologi.
I denne forskning, det lykkedes dem at syntetisere molekylære nanocarboner med knuder og catenaner, som er grundlæggende typer af topologi. Molekyler kaldet catenaner og knob er blevet syntetiseret siden 1960'erne og 1980'erne, henholdsvis. I de seneste år, sådanne molekyler har været forventet at blive anvendt på molekylære maskiner (maskiner i nanoskala) og er velkendte som årsagen til tildelingen af Nobelprisen i kemi i 2016. at generere strukturer af knuder eller catenaner, det var nødvendigt at indføre nitrogenatomer eller oxygenatomer og inducere strukturen til en topologisk struktur ved at bruge sådanne atomer som springbræt. Derfor, en ny syntesemetode skulle udvikles til at syntetisere molekylære nanocarboner med knob eller catenaner.
En molekylær nanocarbon "cycloparaphenylen, "en delvis struktur af et carbon nanorør, er et ringformet molekyle med en diameter på cirka 1 nanometer, der kun består af benzener. De troede, at de ville være i stand til at indføre knuder eller catenaner ved at bruge siliciumatomer som foreløbige fastgørelser i midten af syntetisering af cycloparaphenylener. Da disse siliciumatomer kan fjernes senere, knuder eller catenaner, der kun består af kulstofskeletter, kan opnås i sidste ende.
Først, forberede C-formede molekyler og forbinde midten af to C-formede molekyler med et siliciumatom. Sekund, forbinde enderne af hver af disse C-formede molekyler gennem en reaktion med nikkel bruges til at generere to ringe. Tredje, bruge fluor (tetrabutylammoniumfluorid) til at fjerne siliciumatomet. Endelig, en reaktion med natrium bruges til at omdanne til et molekyle en "al-benzen catenan", hvori to cycloparaphenylener er geometrisk bundet. Med denne syntesemetode det er lykkedes dem at syntetisere catenaner fra et par ringe sammensat af 12 benzener. Ved at bruge en lignende metode, de har syntetiseret 2 milligram catenaner, hvor to ringe af forskellige størrelser er bundet, den ene består af 12 benzener og den anden 9 benzener.
Further applying this synthesis method, they have synthesized topological molecular nanocarbons with knots, "all-benzene knots, " which can be called "unattainable molecules" due to the greater difficulty. As other prior researches have revealed that a topology of molecular knots can be generated by arranging two tentative fastenings at appropriate positions, they designed a precursor with two silicon atoms as the tentative fastenings. They have succeeded for the first time in synthesizing all-benzene knots targeting "carbon knots" by synthesizing molecules in which U-shaped molecules were bound with silicon atoms and then processed with this unit in the same way as all-benzene catenanes (homo-coupling reaction, fluoridization, and sodium reduction reaction). X-ray crystallography was used to confirm that the molecule has a knot. Ud over, they have proved the existence of carbon nanotori (donut-shaped nanocarbons) incorporating the all-benzene knot our research group has synthesized as a partial structure from the standpoint of computational science and have shown that the synthesis of all-benzene knots is an important step toward the synthesis of topological nanocarbons.
Næste, they have proved that those newly synthesized molecules have specific properties derived from the knots or catenanes. It was observed that after catenanes composed of two rings that are different in size are excited by light, the excitation energy is transferred from the larger ring to the smaller ring very rapidly. The catenane structure is the only way to verify the effect of the interaction between the rings with the symmetry of each ring perfectly maintained. The experiments this time have proved that the rings electronically interact via the catenane structure.
På den anden side, when they dissolved all-benzene knots in an organic solvent to conduct NMR measurements of hydrogen nuclei, only one type of signal was observed even at a low temperature of minus 95 degrees. This indicates that signals are leveled due to very rapid motion. A simulation on a supercomputer strongly suggested that such fast-speed levelling is caused by donut-shaped vortex-like motions. They found those properties, which are very difficult to predict in advance, for the first time through synthesis and isolation.
Knots, which can be divided into left-handed ones and right-handed ones, have a property called chirality (e.g. chirality between the left hand and the right hand, which are not equivalent as they are, but become equivalent when reflected by a mirror). They have succeeded in separating the all-benzene knots we synthesized this time into the left-handed knots and right-handed knots and to prove that all-benzene knots show circular dichroism (a phenomenon of different absorption intensities between light rotating in a right-hand sense (right circular polarization) and light rotating in a left-hand sense (left circular polarization), which are observed in molecules with chirality) derived from the chirality of the knots.
Further development
The product of this research will be a big step toward the synthesis of nanocarbons with complex geometric structures. The capability of constructing complex geometric structures such as knots and catenanes from carbon skeletons will lead to the design and synthesis of unprecedented complex nanocarbons. Ud over, the product can be called a monument to be included in textbooks of organic chemistry as an example of synthesizing very beautiful molecules with an innovative method. It is an epoch-making product that can be a starting point of the development of new chemistry because it has the potential to completely change the design of molecular machines based on geometric bond structures.
Sidste artikel3-D printteknik accelererer fremstillingen i nanoskala 1000 gange
Næste artikelMikroskop udskriver mønstre i nanoskala
 Varme artikler
Varme artikler
-
 Ny tilgang giver billeder af enkeltcelle med mikrometeropløsning via kontrast baseret på cellers t…Til venstre, et klassisk fasekontrastbillede af en celle opnået via et standardmikroskop. Til højre, et termisk billede af den samme celle optaget med holdets termiske billedapparat. Kredit:Bordeaux U
Ny tilgang giver billeder af enkeltcelle med mikrometeropløsning via kontrast baseret på cellers t…Til venstre, et klassisk fasekontrastbillede af en celle opnået via et standardmikroskop. Til højre, et termisk billede af den samme celle optaget med holdets termiske billedapparat. Kredit:Bordeaux U -
 Forskere bøjer nanotråde i 2-D og 3-D strukturerDette er et falskfarvet scanningselektronmikroskopbillede af zigzag-nanotrådene, hvor de lige sektioner er adskilt af trekantede led, og specifikke enhedsfunktioner er præcist lokaliseret ved de bøjed
Forskere bøjer nanotråde i 2-D og 3-D strukturerDette er et falskfarvet scanningselektronmikroskopbillede af zigzag-nanotrådene, hvor de lige sektioner er adskilt af trekantede led, og specifikke enhedsfunktioner er præcist lokaliseret ved de bøjed -
 Fotografier af grafen-tænker uden for 2-D-boksenKunstnerisk opfattelse af den foto-termioniske effekt i en grafen-WSe2-grafen heterostruktur. Kredit:© ICFO | Fabien Vialla I et nyligt værk, der blev offentliggjort i Naturkommunikation , en fo
Fotografier af grafen-tænker uden for 2-D-boksenKunstnerisk opfattelse af den foto-termioniske effekt i en grafen-WSe2-grafen heterostruktur. Kredit:© ICFO | Fabien Vialla I et nyligt værk, der blev offentliggjort i Naturkommunikation , en fo -
 Europæiske forskere gør gennembrud i udviklingen af supermateriale grafengrafen, kun et atom tykt, klatrer terrasser på overfladen af et siliconecarbidsubstrat. Dette billede af en grafenenhed blev taget med et atomkraftmikroskop af NPLs Dr. Olga Kazakova (PhysOrg.co
Europæiske forskere gør gennembrud i udviklingen af supermateriale grafengrafen, kun et atom tykt, klatrer terrasser på overfladen af et siliconecarbidsubstrat. Dette billede af en grafenenhed blev taget med et atomkraftmikroskop af NPLs Dr. Olga Kazakova (PhysOrg.co
- AlphaStar er sulten efter verdensherredømme i StarCraft II -kampe
- Vitale infrastrukturer i Holland sårbare over for hackere
- Påvirker musik plantevækst?
- Fleksible solceller:Vil de en dag drive dine enheder?
- Lysemitterende nanorør bliver lysere med nul-dimensionelle tilstande
- Vera Rubin Observatory burde være i stand til at opdage et par interstellare objekter om måneden.


