Diamanter afslører neurale hemmeligheder
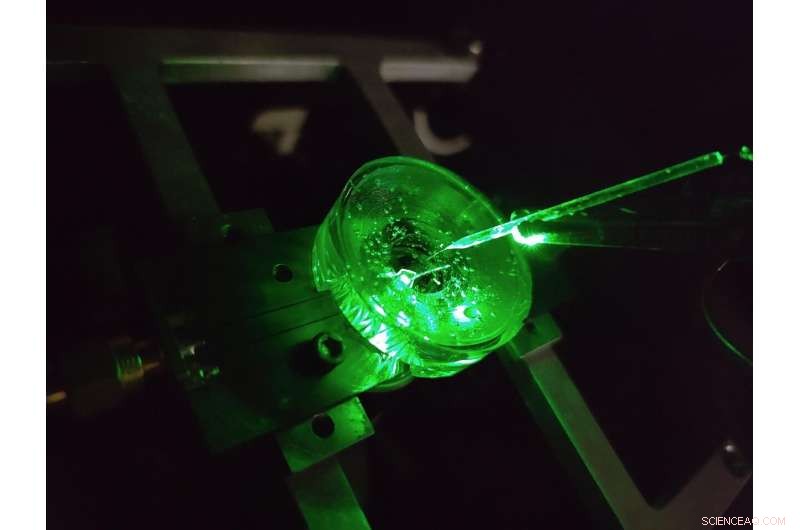
En prototype af et diamantspændingsmikroskop konstrueret af fysikere ved University of Melbourne. En lillebitte elektrode er suspenderet over diamantchippen for at teste enhedens ydeevne. En grøn laser skinnede nedefra giver fluorescensexcitation til chippen. Kredit:Forfatter leveret, University of Melbourne
Hjernen er uden tvivl en af de mest komplekse strukturer i det kendte univers.
Fortsatte fremskridt i vores forståelse af hjernen og vores evne til effektivt at behandle en lang række neurologiske sygdomme er afhængig af at undersøge hjernens neurale mikrokredsløb med stadigt stigende detaljer.
En klasse af metoder til at studere neurale kredsløb kaldes spændingsbilleddannelse. Disse teknikker giver os mulighed for at se den spænding, der genereres af vores hjernes affyrende neuroner – fortæller os, hvordan netværk af neuroner udvikler sig, fungerer og ændrer sig over tid.
I dag udføres spændingsbilleddannelse af dyrkede neuroner ved hjælp af tætte arrays af elektroder, hvorpå celler dyrkes (eller dyrkes), eller ved at anvende lysemitterende farvestoffer, der reagerer optisk på ændringer i spændingen på cellens overflade.
Men det detaljeringsniveau, vi kan se ved brug af disse teknikker, er begrænset.
De mindste elektroder kan ikke pålideligt skelne individuelle neuroner, omkring 20 milliontedele af en meter i diameter, for ikke at sige noget om det tætte netværk af nanoskalaforbindelser, der dannes mellem dem, og der er ikke gjort væsentlige teknologiske fremskridt på dette område i over to årtier.
Desuden kræver hver elektrode sin egen kablede forbindelse og forstærker, hvilket sætter betydelige begrænsninger på antallet af elektroder, der kan måles samtidigt.
Farvestoffer kan overvinde disse begrænsninger ved at afbilde spændingen trådløst som lys – det betyder, at den komplekse elektronik kan placeres væk fra cellerne i et kamera.
Resultatet er høj opløsning over store områder, i stand til at skelne hver enkelt neuron i et stort netværk. Men der er også begrænsninger her. Spændingsreaktionerne for avancerede farvestoffer er langsomme og ustabile.
Vores seneste forskning offentliggjort i Nature Photonics , udforsker en ny type af højhastigheds-, højopløsnings- og skalerbar spændingsbilleddannelsesplatform skabt med det formål at overvinde disse begrænsninger – et diamantspændings-billeddannelsesmikroskop.
Enheden er udviklet af et hold fysikere fra University of Melbourne og RMIT University og bruger en diamantbaseret sensor, der konverterer spændingssignaler på overfladen direkte til optiske signaler – det betyder, at vi kan se elektrisk aktivitet, mens den sker.
The conversion uses the properties of an atom-scale defect in the diamond's crystal structure known as the nitrogen-vacancy (NV).
NV defects can be engineered by bombarding the diamond with a nitrogen ion beam using a special type of particle accelerator. The fabrication of the sensor begins with using this process to create a high-density, ultra-thin layer of NV defects close to the diamond's surface.
You can think of each NV defect as a bucket that holds up to two electrons. When this bucket is empty, the NV defect is dark. With one electron, the NV defect emits orange light when illuminated by a laser—this property is known as fluorescence. With two electrons, the color of the fluorescence becomes red.
A previously discovered property of NV defects is that the number of electrons they hold—and the resulting fluorescence—can be controlled with a voltage. Unlike dyes, the voltage response of an NV defect is very fast and stable.
Our research aims to overcome the challenge of making this effect sensitive enough to image neuronal activity.
On the diamond's surface, the crystal structure ends with a layer one atom thick, made up of hydrogen and oxygen atoms. The NV defects closest to the surface are the most sensitive to changes in voltage outside the diamond, but they are also highly sensitive to the atomic makeup of the surface layer.
Too much hydrogen and the NVs are so dark that the optical signals we are looking for cannot be seen. Too little hydrogen and the NVs are so bright that the small signals we are after are completely washed out.
So, there's a "Goldilocks' zone" for voltage imaging, where the surface has just the right amount of hydrogen.
To reach this zone, our team developed an electrochemical method for removing hydrogen in a controlled way. By doing this, we've managed to achieve voltage sensitivities two orders of magnitude better than what has been previously reported.
We tested our sensor in salty water using a microscopic wire 10-times thinner than a human hair. By applying a current, the wire can produce a small cloud of charge in the water above the diamond. The formation and subsequent diffusion of this charge cloud produces small voltages at the diamond surface.
By capturing these voltages through a high-speed recording of the NV fluorescence, we can determine the speed, sensitivity and resolution of our diamond imaging chip.
We were able to further boost sensitivity by patterning the diamond's surface into 'nanopillars'—conical structures with the NV centers embedded in their tips. These pillars funnel the light emitted by the NVs towards the camera, dramatically increasing the amount of signal we can collect.
With the development of the diamond voltage imaging microscope for detecting neuronal activity, the next step is the recording of activity from cultured neurons in vitro—these are experiments on cells grown outside their normal biological context, otherwise known as test-tube or petri-dish experiments.
What differentiates this technology from existing state-of-the-art in vitro techniques is the combination of high spatial resolution (on the order of a millionth of a meter or less), large spatial scale (a few millimeters in each direction—which for a network of neurons in mammals is quite vast), and complete stability over time.
No other existing system can simultaneously offer these three qualities, and it's this combination that will allow our made-in-Melbourne technology to make a valuable contribution to the work of neuroscientists and neuropharmacologists globally.
Our system will aid these researchers in pursuing both fundamental knowledge and the next generation of treatments for neurological and neurodegenerative diseases. + Udforsk yderligere
New method enables long-lasting imaging of rapid brain activity in individual cells deep in the cortex
 Varme artikler
Varme artikler
-
 Ny terahertz -billeddannelsesmetode kan fremskynde påvisning af hudkræftFor at aktivere højopløselig terahertz -billeddannelse, forskerne brugte et digitalt mikromirrorapparat til at projektere laserlys på en siliciumskive i et specifikt mønster. Når en terahertz -stråle
Ny terahertz -billeddannelsesmetode kan fremskynde påvisning af hudkræftFor at aktivere højopløselig terahertz -billeddannelse, forskerne brugte et digitalt mikromirrorapparat til at projektere laserlys på en siliciumskive i et specifikt mønster. Når en terahertz -stråle -
 Crystal-stacking proces kan producere nye materialer til højteknologiske enhederPræcis epitaksial grænsefladeseparation af PMN-PT på et SRO/STO-substrat. Kredit: Natur (2020). DOI:10.1038/s41586-020-1939-z Det magnetiske, ledende og optiske egenskaber ved komplekse oxider gø
Crystal-stacking proces kan producere nye materialer til højteknologiske enhederPræcis epitaksial grænsefladeseparation af PMN-PT på et SRO/STO-substrat. Kredit: Natur (2020). DOI:10.1038/s41586-020-1939-z Det magnetiske, ledende og optiske egenskaber ved komplekse oxider gø -
 Frustration forklarer forskelle i superledning i molekylære ledere og kopaterFigur 1:Det molekylære arrangement af bis (ethylenedithio) tetrathiafulvalene (BEDT-TTF) laget i molekylærlederen undersøgt i denne undersøgelse (guldkugler:svovl; sølvkugler:kulstof; røde kugler:hull
Frustration forklarer forskelle i superledning i molekylære ledere og kopaterFigur 1:Det molekylære arrangement af bis (ethylenedithio) tetrathiafulvalene (BEDT-TTF) laget i molekylærlederen undersøgt i denne undersøgelse (guldkugler:svovl; sølvkugler:kulstof; røde kugler:hull -
 Hvordan røntgenstråler skubbede topologisk stofforskning over toppenBeamline 10.0.1 på Berkeley Labs Advanced Light Source er optimeret til studier af topologiske egenskaber i materialer. Kredit:Roy Kaltschmidt/Berkeley Lab Mens du bruger røntgenstråler genereret
Hvordan røntgenstråler skubbede topologisk stofforskning over toppenBeamline 10.0.1 på Berkeley Labs Advanced Light Source er optimeret til studier af topologiske egenskaber i materialer. Kredit:Roy Kaltschmidt/Berkeley Lab Mens du bruger røntgenstråler genereret
- USA afbryder russisk botnet på 500, 000 hackede routere
- Sådan beregnes gennemsnitlig brug
- Opmåling af en ø invaderet af tudser og frøer
- Amazon er muligvis klar til at tage imod Apples AirPods, siger rapporten
- Teslas billeverancer stiger, men der er stadig udfordringer
- At passe en biologisk nanopore ind i en menneskeskabt, nye måder at analysere DNA på


