Hvor mange orbitaler er der for hvert niveau og antallet af elektroner?
Energiniveau (n) Orbitaler Elektroner**
-1 1s 2
-2 1'ere, 2'ere, 2p 8
-3 1s, 2s, 2p, 3s, 3p 18
-4 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p 32
-5 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 5s, 4d, 5p 50
-6 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 5s, 5p, 5d, 6s, 4f, 5d, 6p 72
-7 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, 5p, 5d, 5f, 6s, 6p, 6d, 7p 98
-8 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, 5p, 5d, 5f, 5g, 6s, 6p, 6d, 6f, 7s, 7p 128
Sidste artikelHvilken type binding holder baserne parret sammen?
Næste artikelHvad er oxidationstallet for O i CaO?
 Varme artikler
Varme artikler
-
 Mikroenhed kunne opfange tidlige tegn på hjerteanfald eller slagtilfældeKredit:University of Sydney I Australien lider hvert år cirka 55.000 mennesker af et hjerteanfald, hvoraf et tilsvarende antal lider af slagtilfælde. Mange er forårsaget af blodpropper, der blokere
Mikroenhed kunne opfange tidlige tegn på hjerteanfald eller slagtilfældeKredit:University of Sydney I Australien lider hvert år cirka 55.000 mennesker af et hjerteanfald, hvoraf et tilsvarende antal lider af slagtilfælde. Mange er forårsaget af blodpropper, der blokere -
 XenonPy.MDL:Et omfattende bibliotek af fortrænede modeller til materialeegenskaberTermofysiske egenskaber (dvs. termisk ledningsevne) af polymerer forudsagt af transfer learning (TL). Den fælles forskergruppe lykkedes med at konstruere en maskinlæringsmodel, der er i stand til ekst
XenonPy.MDL:Et omfattende bibliotek af fortrænede modeller til materialeegenskaberTermofysiske egenskaber (dvs. termisk ledningsevne) af polymerer forudsagt af transfer learning (TL). Den fælles forskergruppe lykkedes med at konstruere en maskinlæringsmodel, der er i stand til ekst -
 Kompleks liv udviklede sig ud af den tilfældige kobling af små molekylerEt simpelt RNA-molekyle som dette kan have været ansvarlig for udviklingen af komplekst liv, som vi kender det. Kredit:Wits University Kompleks liv, som vi kender det, startede helt tilfældigt,
Kompleks liv udviklede sig ud af den tilfældige kobling af små molekylerEt simpelt RNA-molekyle som dette kan have været ansvarlig for udviklingen af komplekst liv, som vi kender det. Kredit:Wits University Kompleks liv, som vi kender det, startede helt tilfældigt, -
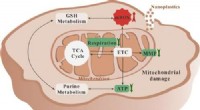 Nanoplast kan forstyrre menneskelige lever- og lungecelleprocesser i laboratorieeksperimenterGrafisk abstrakt. Kredit:Environmental Science &Technology (2022). DOI:10.1021/acs.est.2c03980 Hvad sker der, når folk ubevidst spiser, drikker eller inhalerer næsten usynlige plastikstykker? Selvo
Nanoplast kan forstyrre menneskelige lever- og lungecelleprocesser i laboratorieeksperimenterGrafisk abstrakt. Kredit:Environmental Science &Technology (2022). DOI:10.1021/acs.est.2c03980 Hvad sker der, når folk ubevidst spiser, drikker eller inhalerer næsten usynlige plastikstykker? Selvo
- Største brand vokser, tvinger evakuering af dyrelivsstation
- EU-virksomheder slår ud mod nye net-privatlivsregler
- Sådan bygges en seismograf
- Ny teknologi gør en solrig dag til sikkert vand
- Forskere skaber en mere nøjagtig model for, hvordan nogle mikrober søger efter næringsstoffer
- Tre typer stenarter, der formes, når Lava Cools


