Sådan beregnes nej. af atomer hydrogen carbon oxygen nitrogen til stede i 5,6 gram urinstof?
1. Bestem den molære masse af urinstof:
* Carbon (c):12,01 g/mol
* Hydrogen (H):1,01 g/mol
* Nitrogen (n):14,01 g/mol
* Oxygen (O):16,00 g/mol
Molær masse urinstof (ch₄n₂o) =(12,01) + (4 * 1,01) + (2 * 14,01) + (16,00) =60,06 g/mol
2. Beregn molen af urinstof:
* Mol =masse / molær masse
* Mol urinstof =5,6 g / 60,06 g / mol =0,0932 mol
3. Beregn antallet af molekyler:
* Avogadros nummer =6,022 x 10²³ molekyler/mol
* Antal urinstofmolekyler =0,0932 mol * 6,022 x 10²³ molekyler/mol =5,61 x 10²² molekyler
4. Beregn antallet af atomer for hvert element:
* brint (H): Hvert urinstofmolekyle har 4 hydrogenatomer.
* Antal H -atomer =5,61 x 10²² molekyler * 4 atomer/molekyle =2,24 x 10²³ atomer
* carbon (c): Hvert urinstofmolekyle har 1 carbonatom.
* Antal C -atomer =5,61 x 10²² molekyler * 1 atom/molekyle =5,61 x 10²² atomer
* nitrogen (n): Hvert urinstofmolekyle har 2 nitrogenatomer.
* Antal N -atomer =5,61 x 10²² molekyler * 2 atomer/molekyle =1,12 x 10²³ atomer
* ilt (O): Hvert urinstofmolekyle har 1 iltatom.
* Antal O -atomer =5,61 x 10²² molekyler * 1 atom/molekyle =5,61 x 10²² atomer
Derfor:
* brint: 2.24 x 10²³ atomer
* carbon: 5,61 x 10²² atomer
* nitrogen: 1,12 x 10²³ atomer
* ilt: 5,61 x 10²² atomer
 Varme artikler
Varme artikler
-
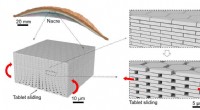 Ny type glas inspireret af naturen er mere modstandsdygtig over for stødMikroarkitekturen af Nacre og tablet, der glider i Nacre. Kredit:Z. Yin, F. Hannard, F. Barthelat Ved at bruge den iriserende perlemor, der ofte findes foring af muslingeskaller, forskere har ko
Ny type glas inspireret af naturen er mere modstandsdygtig over for stødMikroarkitekturen af Nacre og tablet, der glider i Nacre. Kredit:Z. Yin, F. Hannard, F. Barthelat Ved at bruge den iriserende perlemor, der ofte findes foring af muslingeskaller, forskere har ko -
 Isolerende mursten med mikroskopiske boblerFor at opnå de samme isoleringsværdier som en 165 mm tyk væg af aerobricks, en mur af perlitsten skal være 263 mm tyk — og en mur af ikke-isolerende mursten skal være mere end en meter. Kredit:Empa
Isolerende mursten med mikroskopiske boblerFor at opnå de samme isoleringsværdier som en 165 mm tyk væg af aerobricks, en mur af perlitsten skal være 263 mm tyk — og en mur af ikke-isolerende mursten skal være mere end en meter. Kredit:Empa -
 Mikrobølger afslører detaljeret struktur af molekylær motorSom en makroskopisk motor, det kunstige motormolekyle har en stator (nederst) og en rotor (øverst), forbundet med en aksel. Kredit:Sérgio Domingos / DESY Et team af forskere har brugt mikrobølger
Mikrobølger afslører detaljeret struktur af molekylær motorSom en makroskopisk motor, det kunstige motormolekyle har en stator (nederst) og en rotor (øverst), forbundet med en aksel. Kredit:Sérgio Domingos / DESY Et team af forskere har brugt mikrobølger -
 Efterligner biologisk proces, hydrogel signalerer og frigiver proteinerEt syntetisk væv frigiver terapeutiske proteiner (rødbrun/gul), når de er udløst af metabolitter (sandbrun). Metabolitterne kommer i kontakt med det dobbeltstrengede DNA (rød/blå) for at frigive det r
Efterligner biologisk proces, hydrogel signalerer og frigiver proteinerEt syntetisk væv frigiver terapeutiske proteiner (rødbrun/gul), når de er udløst af metabolitter (sandbrun). Metabolitterne kommer i kontakt med det dobbeltstrengede DNA (rød/blå) for at frigive det r
- Anvendelse af ny teknologi til at løse miljømæssige udfordringer
- Forskere finder ud af, at tang hjælper med at fange kuldioxid i sediment
- Skolepsykologer udvikler intervention for at reducere forstyrrelser på gangen
- NASA-satellitten fanger slutningen på den post-tropiske Storm Kyle
- Hvorfor Venus roterer langsomt på trods af solens kraftfulde greb:Planetens atmosfære forklarer si…
- For at gøre søer sunde, du har først brug for den rigtige opskrift


