Hvordan beregner du atomprocenten af forbindelser?
1. Bestem den kemiske formel:
* Du har brug for den kemiske formel for forbindelsen. For eksempel:
* Vand (H₂O)
* Carbon dioxide (CO₂)
* Sodium chloride (NaCl)
2. Find the Number of Atoms of Each Element:
* Count the number of atoms of each element in the formula.
* Water (H₂O):2 Hydrogen atoms, 1 Oxygen atom
* Carbon dioxide (CO₂):1 Carbon atom, 2 Oxygen atoms
* Sodium chloride (NaCl):1 Sodium atom, 1 Chlorine atom
3. Calculate the Total Number of Atoms:
* Add up the number of atoms of all elements in the formula.
* Water:2 + 1 =3 atoms
* Carbon dioxide:1 + 2 =3 atoms
* Sodium chloride:1 + 1 =2 atoms
4. Calculate the Atomic Percentage:
* For each element, divide the number of atoms of that element by the total number of atoms in the compound, and multiply by 100%.
* Vand:
* Hydrogen:(2 / 3) * 100% =66.67%
* Oxygen:(1 / 3) * 100% =33.33%
* kuldioxid:
* Carbon:(1 / 3) * 100% =33.33%
* Oxygen:(2 / 3) * 100% =66.67%
* natriumchlorid:
* Sodium:(1 / 2) * 100% =50%
* Chlorine:(1 / 2) * 100% =50%
Vigtig note: Atomic percentage is a measure of the relative number of atoms of each element in a compound. It's different from the mass percentage, which considers the mass of each element in the compound.
 Varme artikler
Varme artikler
-
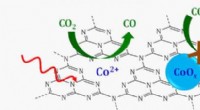 Lysaktiveret, single-ion katalysator nedbryder kuldioxidSkematisk over en enkeltstedskatalysator, hvor enkelte koboltioner (CO 2 +) understøttet på et grafitisk kulstofnitrogenlag (C3N4) reducerer kuldioxid (CO 2 ) til carbonmonoxid (CO) i nærvær af sy
Lysaktiveret, single-ion katalysator nedbryder kuldioxidSkematisk over en enkeltstedskatalysator, hvor enkelte koboltioner (CO 2 +) understøttet på et grafitisk kulstofnitrogenlag (C3N4) reducerer kuldioxid (CO 2 ) til carbonmonoxid (CO) i nærvær af sy -
 Sikker udskrivning med vandbaseret usynligt blækForskere i Kina har udviklet en omskrivbar papirbelægning, der kan kryptere hemmelig information med relativt lavteknologisk usynligt blæk-vand. En besked, der udskrives af en vandstråleprinter på et
Sikker udskrivning med vandbaseret usynligt blækForskere i Kina har udviklet en omskrivbar papirbelægning, der kan kryptere hemmelig information med relativt lavteknologisk usynligt blæk-vand. En besked, der udskrives af en vandstråleprinter på et -
 Forskere belyser krystalstrukturen af natriumboridSkoltech Professor Artem Oganov Kredit:Skoltech Et internationalt hold af videnskabsmænd sammen med professor Artem Oganov fra Skoltech og Moskva Institut for Fysik og Teknologi rapporterer om den
Forskere belyser krystalstrukturen af natriumboridSkoltech Professor Artem Oganov Kredit:Skoltech Et internationalt hold af videnskabsmænd sammen med professor Artem Oganov fra Skoltech og Moskva Institut for Fysik og Teknologi rapporterer om den -
 Den perfekte blanding:Optimering af gasblandinger til brintlagring i clathrathydraterForskere finder optimal hydrogen-naturgasblanding til at fange brint i burlignende molekyler mere effektivt. Kredit:GIST I vores igangværende søgen efter at forvandle sig til et mere miljøvenligt
Den perfekte blanding:Optimering af gasblandinger til brintlagring i clathrathydraterForskere finder optimal hydrogen-naturgasblanding til at fange brint i burlignende molekyler mere effektivt. Kredit:GIST I vores igangværende søgen efter at forvandle sig til et mere miljøvenligt
- Laver calcium og svovl en kovalent binding?
- Hvordan kommer forskere på spørgsmål?
- For at forbedre undersøgelser og anden forskning studerer entomologer, hvordan farver tiltrækker v…
- Mød stenaldertrønderen
- Mikroplast kan påvirke, hvordan arktisk havis dannes og smelter
- Ny eksotisk stofpartikel, en tetraquark, opdaget


