At se nanoteknologiens verden fra et enkelt-molekyle perspektiv
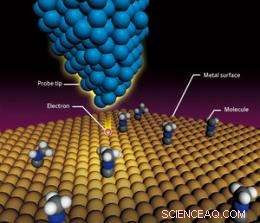
Princippet om scanning tunneling mikroskopi. Når en spænding påføres en atomisk skarp STM-spids, der bringes tæt på et molekyle på en metaloverflade, en tunnelstrøm løber mellem spidsen og molekylet, at injicere elektroner i molekylet og inducere en molekylær vibration. Intensiteten af den molekylære vibration ved en given spænding kan bruges til at identificere molekylet. Denne teknik kan også bruges til at fremkalde en kemisk reaktion. Copyright:RIKEN
At observere strukturen af kollapsende ustabile atomkerner ved hjælp af elektroner er et eksperimentelt mål, som ikke er blevet opnået nogen steder i verden. Masanori Wakasugi, direktør for Instrumentation Development Group på RIKEN Nishina Center for Accelerator-Based Science (RNC), arbejder på denne udfordrende problemstilling.
Den nuværende teoretiske model af atomkernen er blevet konstrueret med store bidrag fra elektronspredningsforsøg, hvor elektroner kolliderer med stabile atomkerner for at visualisere kernestrukturen. I de seneste år, imidlertid, en lang række eksperimenter med ustabile atomkernes egenskaber har afsløret en række fænomener, som ikke stemmer overens med den nuværende model for atomkernen.
Radioisotop-elektronspredningsforsøg, hvor elektroner kolliderer med ustabile kerner, er uundværlige for at etablere den ultimative model for atomkernen, hvilket vil give en omfattende forståelse af både stabile og ustabile kerner. Wakasugi og hans kolleger tager unikke tilgange til at opnå dette verdensførste eksperiment.
Observere de kemiske reaktioner af enkelte molekyler
"Da jeg gik i ungdomsskolen, Jeg lærte den kemiske formel for elektrolyse af vand, siger Kim. Denne formel er H2O → H2 + 1/2O2. "Jeg spurgte min lærer, hvorfor vi skal gange O2 med det halve. Læreren svarede, at ilten ganges med det halve, fordi når vandet elektrolyseres, brint og ilt produceres i forholdet to til én. Imidlertid, Jeg troede, hvad hvis et enkelt vandmolekyle elektrolyseres? Dette spørgsmål gav mig incitamentet til at observere processen med en kemisk reaktion på skalaen af et enkelt molekyle."
Kim gik videre til Institut for Kemi ved Seoul National University, hvor han studerede elektrokemi. "På det tidspunkt Jeg udførte eksperimenter, der brugte et elektrisk kredsløb, som ved elektrolyse af vand, at kontrollere en kemisk reaktion i en opløsning og at undersøge den kemiske reaktion fra reaktionsprodukterne. Denne tilgang, imidlertid, giver ikke oplysninger om, hvordan enkelte molekyler er involveret i en kemisk reaktion. Vi kan kun gætte."
Efter at have afsluttet sin kandidatuddannelse ved Seoul National University, han besøgte Japan i 1996 og startede forskning ved University of Tokyo under opsyn af Akira Fujishima, nu præsident for Tokyo University of Science, som var kendt som 'fotokatalysatorens fader'. Fotokatalyse er en proces, hvorved molekyler kan nedbrydes på overfladen af et fotoaktivt materiale, såsom titaniumoxid, ved udsættelse for lys. "Jeg havde oprindeligt planer om at lave en grundig undersøgelse af fotokatalysatorer. Imidlertid, Prof. Fujishima foreslog, at jeg lavede mere grundlæggende forskning, fordi min baggrund var inden for naturvidenskab. Så jeg besluttede at studere de fysiske fænomener, der opstår, når overfladen af et stof udsættes for lys."
Reagerer et enkelt molekyle
"Da jeg var på tredje år af mit ph.d.-program, Jeg stødte på et meget spændende papir, der rapporterede, at et scanningstunnelmikroskop med succes var blevet brugt til at observere den 'molekylære vibration' af et enkelt molekyle. Jeg tænkte med det samme, at det var det, jeg virkelig ville gøre.”
Et scanning tunneling mikroskop (STM) er en billeddannelsesteknik, der gør det muligt at kortlægge den mikroskopiske overfladestruktur af et stof ved opløsninger, der nærmer sig skalaen af individuelle atomer. Men dette er ikke den eneste funktion af STM; det kan også bruges til at identificere de tilstedeværende typer af molekyler baseret på den molekylære vibration.
I STM, en spænding påføres en meget skarp sondespids, der bringes meget tæt på et molekyle på en overflade. Elektroner fra sonden strømmer til målmolekylet, producerer det, der kaldes en 'tunnelstrøm', henviser til den måde, elektroner ser ud til at 'tunnelere' gennem den klassiske energibarriere, der er nødvendig for at sådan en strøm kan flyde. Denne strøm inducerer en molekylær vibration, hvilket får alle de individuelle atomer i målmolekylet til at blive fortrængt fra deres ligevægtspositioner. Intensiteten af den molekylære vibration svarende til en given spænding afhænger af typen af molekyle eller de kemiske bindinger i molekylet. Typen af molekyle kan derfor identificeres ved at observere den molekylære vibration.
"Jeg ledte efter et forskningslaboratorium, hvor jeg kunne bruge STM i Japan, da prof. Fujishima introducerede mig til Surface Chemistry Laboratory på RIKEN, ledet på det tidspunkt af chefforsker Maki Kawai, som nu er administrerende direktør for RIKEN."
Efter at have tilsluttet sig Surface Chemistry Laboratory i 1999, Kim udviklede STM-teknologier sammen med Tadahiro Komeda, en forsker i laboratoriet og nu professor ved Tohoku University. der, Kim observerede molekylære vibrationer for at kunne identificere individuelle molekyler på dette grundlag. Det lykkedes ham også at injicere elektroner i et bestemt sted på et molekyle, dermed ændre det til et andet molekyle.
"Vi fjernede to brintatomer fra et trans-2-butenmolekyle bestående af fire kulstof- og otte brintatomer for at producere en 1, 3-butadien-molekyle bestående af fire carbon- og seks hydrogenatomer. Vi brugte STM til at forårsage en kemisk reaktion som tilsigtet i et enkelt molekyle, observerede vibrationssignalerne før og efter reaktionen, og identificerede typen af molekyle med succes for første gang."
Kim tilskriver succesen med at fremkalde den ønskede kemiske reaktion til laboratoriets tidligere arbejde med katalyse. "Vi placerede et molekyle på overfladen af palladium, som fungerede som katalysator for den kemiske reaktion. Surface Chemistry Laboratory startede oprindeligt som et katalysatorforskningslaboratorium, og vi skylder meget den enorme ophobning af viden om molekyler og katalysatorer på overfladen af stoffer."
Styring af individuelle molekyler
Der var stadig en teknisk udfordring at overvinde ved at observere molekylære vibrationer ved STM. "Når elektroner injiceres fra en STM-sondespids ind i et molekyle, nogle molekyler begynder at bevæge sig, før deres molekylære vibrationer observeres. At finde en effektiv måde at observere disse ustabile molekyler var et stort problem for os."
Kim og hans laboratoriekolleger undersøgte, hvilket elektronenerginiveau der får molekylet til at bevæge sig. "Som et resultat, vi fandt ud af, at molekylet bevæger sig ved et indsprøjtet elektronenerginiveau svarende til det, der forårsager den stærkeste molekylære vibration." Baseret på disse eksperimenter, de etablerede en unik målemetode kaldet 'aktionsspektroskopi'. "Denne målemetode gjorde det muligt for os at identificere alle typer molekyler, både stabile og ustabile molekyler, og at undersøge deres væsentlige egenskaber."
Når elektroner injiceres fra en STM-sondespids ind i et molekyle, molekylet kan bevæge sig i mange retninger. "Vi kan ikke kontrollere retningen af et molekyles bevægelse, men vi støder kun på dette problem, når STM-probespidsen er placeret lige over molekylet. So we placed the STM probe tip obliquely upward and used the electrostatic force acting between the probe tip and the molecule. This approach also enabled us to control the direction of movement of the molecule successfully.
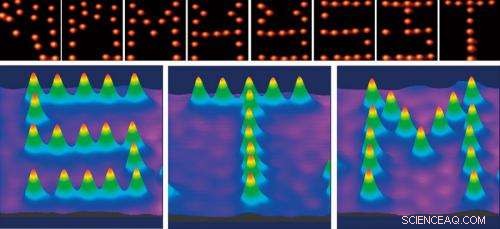
Letters drawn using an STM tip to move molecules. Electrostatic force between organic molecules (CH3S) and the STM tip was used to move the organic molecules to form the letters S, T and M (lower). The upper pictures show the drawing process for each letter. Copyright :RIKEN
Kims team has used this technique to draw letters by moving molecules. I slutningen af 1980'erne, a paper was published describing an experiment in which the atoms forming a molecule were moved by STM to construct letters. I det forsøg, the letters were created by drawing the atoms closer to the probe tip or by using the tip to shape the atoms. We constructed our letters by moving the molecules themselves in the desired direction on a surface. This cannot be achieved without a complete understanding of the nature of molecules and the interaction between electrons and molecules. In the future, this technique will be applied in the fabrication of computer circuits by arranging molecules.
Electrolyzing single water molecules
I 2009 Kim started the experiment that he first imagined when he was in junior high schoolthe experiment to electrolyze a single water molecule. In electrolyzing a single water molecule, there are two possible reaction pathways, ” siger han. Those pathways are H2O → 2H + O, and H2O → H + OH. In the former reaction, the two hydrogen atoms are separated from the single oxygen atom, and can be achieved by injecting electrons with high energy. The difficulty is how to produce the other reaction pathway.
Electrons injected into a molecule from an STM tip cause the molecule to start vibrating in an excited state. If the duration of the excited state (vibrational lifetime) is long enough, the molecular vibration causes the bonds between the atoms to break down, which increases the probability of a chemical reaction occurring. When a single water molecule is placed on the surface of a metal, the water molecule cannot be broken down because of its short vibrational lifetime. This is because the water molecule binds chemically to the metal surface, and the energy of the injected electrons is easily dissipated into the metal surface.
Placing a water molecule on the surface of an insulator instead of a metal can increase the vibrational lifetime because no chemical reactions can occur and no electronic energy is absorbed. Imidlertid, a tunneling current cannot flow from the STM probe tip in this case because the water molecule is on an insulator. To cope with this problem, we developed a metal surface coated with an ultrathin film of magnesium oxide just two atoms thick. A water molecule on this surface produces a small tunneling current in STM.
Teoretisk set, a water molecule can be electrolyzed when injected with an electron having an energy of 0.77 electronvolts or more. On the ultrathin MgO film, imidlertid, the water molecule broke down at just 0.45 electronvolts. We attributed this to a multi-step excitation process in which the water molecule is excited by the first injected electron and then by the following injected electron while the water molecule is still in the vibrationally excited state, because the electron energy is slowly dissipated owing to the ultrathin insulating film surface and hence the vibrational lifetime is increased.
The results of their experiments showed exactly what they were looking for. Using this approach, we succeeded in separating a single hydrogen atom from a single water molecule, says Kim. These results confirmed the H2O → H + OH reaction pathway experimentally for the first time, and could lead to the development of technologies for producing hydrogen fuel with the minimum consumption of energy.
Practical applications of single-molecule experiments
I 2010 Kim started the Surface and Interface Science Laboratory at the RIKEN Advanced Science Institute. We are working on new research into the interaction between light and substances. Many researchers have already investigated this subject. Imidlertid, there have been virtually no reports on experiments that examine the interaction between light and substances while observing individual molecules.
Photocatalysts are a firm research target. In Prof. Fujishimas laboratory, I used to watch how he advanced his own research into photocatalysts around him. Denne gang, I intend to conduct research into the essence of photocatalysts in my own right based on the technology and experience I gained over the years at RIKEN.
On a single-molecular scale, nobody knew the position on titanium oxide at which a photocatalytic reaction occurs. It has been considered for years that the photocatalytic reaction occurs at positions where oxygen atoms are missing on the surface of titanium oxide because electrons concentrate at those positions. Our experiments with an STM probe tip clarified that photocatalytic reactions actually occur across wide electronically active areas around the positions where oxygen atoms are missing.
The Surface and Interface Science Laboratory is also conducting research into organic solar cells. What types of molecules are most effective and how should we arrange them to increase power generation efficiency? Many researchers from around the world have wanted to perform single-molecule experiments while observing individual molecules, but such experiments have been too difficult to handle. We have accumulated STM technology that I am confident will enable such experiments.
Toward sci-engineering
"Hidtil har I have focused on research into the essence of chemistry. In the future I also plan to start research that helps us link that knowledge to practical applications. This idea was triggered by a meeting with Dr Takanori Fukushima from the Energy Conversion Research Team. He specializes in organic synthesis and can synthesize any organic molecule. I always have a good time with him, talking about our dreams.
Molecules and matter exhibit different characteristics on the nanometer or molecular scale compared with the macroscale behavior scientists are most familiar with. This is the reason for the widespread scientific interest in nanotechnology over the past ten years, and the origin of the expectations for a nanotechnology revolution.
These expectations, imidlertid, are now on the point of fading because the findings to date have fallen short of societys expectations. Although many theoretical papers have been published on what is actually going on in the nanometer world, only a few study have been reported because of the technical difficulty in directly observing the nature and functions of individual molecules. Many conventional application studies have been conducted without fully understanding the basic mechanisms of nanotechnology. I plan to make use of the STM to study the nature of individual molecules and open a new frontier in nanoscience that will allow us to explore the essence of the nanoworld.
RIKEN launched systematic research into nanoscience before anywhere else in the world, Kim points out. In 1993, Dr Kawai, now an Executive Director of RIKEN, started the Atomic Scale Sci-engineering Research and Promotion Group together with Chief Scientist Masakazu Aono, now a fellow at the National Institute for Materials Science, and Chief Scientist Katsunobu Aoyagi, who is now professor at Ritsumeikan University. Sci-engineering is a term implying that research into the essence of a phenomenon should come first, and then engineering should follow from the results. I would like to follow the research concept of sci-engineering in the Surface and Interface Science Laboratory.
Sidste artikelGrøn nanosyn er nu en køreplan for udvikling
Næste artikelNanobundter giver et kraftfuldt slag
 Varme artikler
Varme artikler
-
 Nanopartikel-jamming ved vand-olie-grænsefladenFastgørelse af NPer til tyndt befolket vand-olie-grænseflade. (A) Skematisk diagram, der viser fastgørelsen af en NP til den uberørte vand-olie-grænseflade. (B) Konfokale mikroskopibilleder, der vis
Nanopartikel-jamming ved vand-olie-grænsefladenFastgørelse af NPer til tyndt befolket vand-olie-grænseflade. (A) Skematisk diagram, der viser fastgørelsen af en NP til den uberørte vand-olie-grænseflade. (B) Konfokale mikroskopibilleder, der vis -
 Nyudviklet model af DNA kaster lys over molekylers fleksibilitetViden om, hvordan DNA folder og bøjer kan give et nyt perspektiv på, hvordan det håndteres i celler, samtidig med at det hjælper med design af DNA-baserede enheder i nanoskala, siger en biomedicinsk i
Nyudviklet model af DNA kaster lys over molekylers fleksibilitetViden om, hvordan DNA folder og bøjer kan give et nyt perspektiv på, hvordan det håndteres i celler, samtidig med at det hjælper med design af DNA-baserede enheder i nanoskala, siger en biomedicinsk i -
 Dræber kræft i øjeblikketLevering og aktivering af gener af guldnanoroder. Guldnanoroder belagt med ladede lipider binder effektivt til DNA og trænger ind i celler. Teamet designede et kunstigt gen, der tændes af varme genere
Dræber kræft i øjeblikketLevering og aktivering af gener af guldnanoroder. Guldnanoroder belagt med ladede lipider binder effektivt til DNA og trænger ind i celler. Teamet designede et kunstigt gen, der tændes af varme genere -
 Forskere afslører synergistiske virkninger i dobbelt enkeltatoms katalysatorSynergistiske virkninger for forbedret katalyse i en dobbelt atom-katalysator. Kredit:FU Junhong Single-atom katalysatorer (SACer) anvendes i heterogen katalyse. I stedet for én type enkelt atom,
Forskere afslører synergistiske virkninger i dobbelt enkeltatoms katalysatorSynergistiske virkninger for forbedret katalyse i en dobbelt atom-katalysator. Kredit:FU Junhong Single-atom katalysatorer (SACer) anvendes i heterogen katalyse. I stedet for én type enkelt atom,
- Optiske teknikker giver hurtige, effektiv COVID-19-detektion
- Spordampgenerator til detektering af sprængstoffer, narkotika
- Forskellige typer af pendler
- Er din familie CO sikker, når store storme rammer?
- Forskere undersøger måder at fjerne antibiotika, der forurener søer og floder
- Ariane 5s anden lancering i 2019


