Hemmeligheden bag cellevækst kunne ligge i yo-yo og gear-lignende tendenser
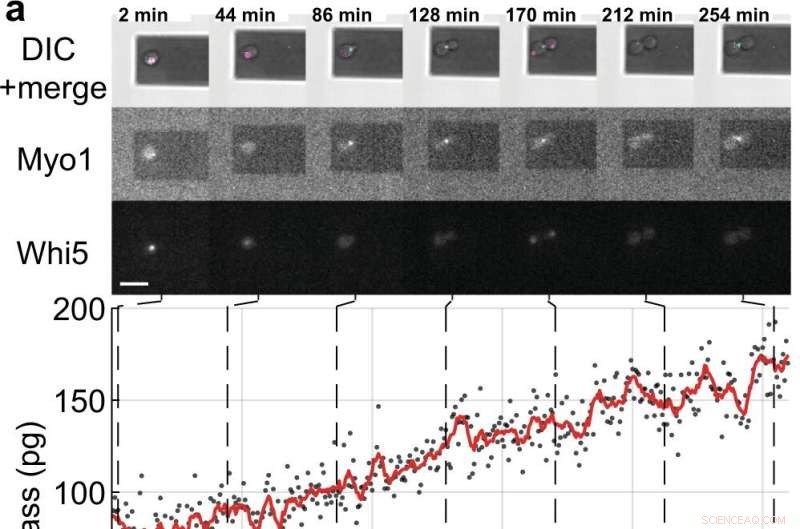
Masse- og cellecyklusmålinger af enkelte S. cerevisiae-celler spirende datterceller. a, b Enkeltgærceller, der udtrykker de fluorescensmærkede cellecyklusmarkørproteiner (Myo1-mKate2 (3×) og Whi5-mKOκ (1×)), blev afbildet ved hjælp af differentiel interferenskontrast (DIC) og fluorescensmikroskopi hver 2 min (øverste paneler ). En fase- og amplitudekurve for mikrocantileveren blev optaget over intervaller ≈50 s for at måle cellemassen ved hjælp af sweep-tilstanden (Supplerende film 4). Mellem på hinanden følgende massemålinger blev de infrarøde og blå lasere i picobalancen slukket i ≈ 20 s for at reducere blegning af fluoroforerne og for at reducere potentiel forstyrrelse af gærvækst. Cellemasseværdier afledt af sæt af enkelte amplitudekurver er vist som grå prikker. Gennemsnitlige rådata (350 s bevægende vindue, rød linje) viser tendensen. Cyan søjler på tidsaksen angiver S/G2/M fasen af gærcellecyklussen, og magenta søjler angiver G1 fasen. Stjernen (*) i b betegner (delvis) løsrivelse af dattercellen efter cytokinese, hvilket falder den totale masse. Skala søjler (hvide), 10 µm. c Vækstkurver for (n =19) enkelte gærceller, der skrider frem gennem S/G2/M-fasen (knopvækst) målt ved picobalancen ved brug af sweep-tilstanden i (n =19) uafhængige eksperimenter. De samlede væksthastigheder mellem start- og slutmasse varierer mellem 0,1 og 2,0 pg min –1 , med et gennemsnit på 0,7 ± 0,5 pg min –1 (gennemsnit ± SD). Varigheden af S/G2/M-fasen varierer fra 57 til 184 min. med et gennemsnit på 96 ± 35 min. Kredit:Nature Communications (2022). DOI:10.1038/s41467-022-30781-y
Celler, de mest basale enheder af livet, der danner alle levende organismer, har længe vogtet på deres hemmeligheder, men nu har et internationalt hold fra University of Sydney, ETH Zürich og University of Basel afsløret nogle af deres hemmeligheder gennem udviklingen af en verden -første teknik.
Forskere ved, at celler vokser, men det var almindeligt antaget, at de voksede lineært eller eksponentielt i størrelse, før de deler sig.
Nu i et papir offentliggjort i Nature Communications ledet af University of Sydney fysiker Dr. David Martinez-Martin, ved hjælp af en nanoteknologisk teknik kaldet "inertiel picobalance", har forskere identificeret, at på enkeltcelleniveau vokser gær i sekventielle intervaller eller segmenter af lineær vækst (konstant væksthastighed) . Ved hvert interval skifter gærceller til hurtigere eller langsommere vækst - en "gear-lignende" tendens.
Forskningen blev udført med saccharomyces cerevisiae, en encellet gærorganisme, der er fundamental i produktionen af brød, øl, vin og lægemidler. De proteinkodende gener fra mange typer gær afspejler gener i dyreceller, hvilket gør dens adfærd nøglen til at forstå menneskelig sygdom.
Det er bemærkelsesværdigt, at den adfærd, der findes i gær, adskiller sig væsentligt fra den hos dyreceller (inklusive mennesker). It was not until 2017 that Dr. Martinez-Martin and colleagues, also using picobalance, first observed that the mass of living mammalian cells fluctuate intrinsically—they "yo-yo" in size.
"We have uncovered processes that challenge models in biology that have been central for decades," said Dr. Martinez-Martin. "The behaviors we have identified in cells from fungus and animal kingdoms provide strong evidence that cells have different strategies to regulate their mass and size, paving the way to better understand how they can accurately form and reform complex structures such as the eyes, brain and fingers in our bodies."
A recent mathematical model published in Journal of Biological Research—Thessaloniki by Dr. Martinez-Martin also offers fresh insight into the meaning of this once-secretive cellular flux.
"Another of our recent studies has found that while cell mass fluctuations have been detected in single mammalian cells, they can be perfectly viable in organisms comprised of many mammalian cells, including humans. Our modeling suggests that the body's cells don't all swell and decrease at the same time—instead they give and take from each other, maintaining an adequate distribution of the body's mass and volume.
"Mass fluctuations may be used by cells to regulate cellular functions such as metabolism, gene expression, proliferation and cell death, by means of altering the concentration and crowdedness of chemical cellular components."
The model also suggests that mass fluctuations allow cells to communicate, both by acting as biomechanical signals through volume fluctuations, and through the exchange of water and molecules.
"I believe this could be a fundamental mechanism which may help cells locate and communicate their position within an organism," Dr. Martinez-Martin said. "Therefore, it could be incredibly important, because it could allow cells to identify and serve their distinct role and purpose in the body."
"Researchers believe that a better understanding of how cells change their mass and size over time, as well as dysregulation of this process (when cells change their size atypically), could be the key to developing the next generation of diagnostics and treatments for a range of diseases, such as cancer, diabetes and cardiovascular disease."
About inertial picobalance:The technique used in the discovery
Dr. Martinez-Martin, who has been recently distinguished by the World Intellectual Property Organization as a young change maker, is the principal inventor of inertial picobalance, a new technology that measures the mass of single or multiple living cells in real-time, enhancing the understanding of cell physiology. The technology is currently being commercialized by the Swiss nanotech company, Nanosurf AG.
In a Nature paper published in 2017, using inertial picoblance, Dr. Martinez-Martin and his colleagues discovered that the mass of living mammalian cells fluctuates intrinsically by one to four percent over seconds, largely due to water entering and exiting cells.
Using this technique, they were also able to observe cells infected with the vaccinia virus (a virus from the poxvirus family). The infected cells showed different mass behavior over time than non-infected cells, potentially enabling a new way of detecting viral infections. + Udforsk yderligere
Getting bacteria and yeast to talk to each other, thanks to a 'nanotranslator'
 Varme artikler
Varme artikler
-
 At finde nano-nålen i høstakkenNanosølv bruges i forskellige applikationer såsom antibakterielle bandager. (Foto:Shutterstock) Norske forskere er blandt de første i verden til at bruge radioaktivitet til at spore nanopartikler
At finde nano-nålen i høstakkenNanosølv bruges i forskellige applikationer såsom antibakterielle bandager. (Foto:Shutterstock) Norske forskere er blandt de første i verden til at bruge radioaktivitet til at spore nanopartikler -
 Team udvikler hurtig SARS-CoV-2-test baseret på ny plasmonisk-fluor biomærkningsteknologiIngeniører ved McKelvey School of Engineering ved Washington University i St. Louis har modtaget føderal finansiering til en hurtig COVID-19-test ved hjælp af en nyudviklet teknologi kaldet plasmonic-
Team udvikler hurtig SARS-CoV-2-test baseret på ny plasmonisk-fluor biomærkningsteknologiIngeniører ved McKelvey School of Engineering ved Washington University i St. Louis har modtaget føderal finansiering til en hurtig COVID-19-test ved hjælp af en nyudviklet teknologi kaldet plasmonic- -
 Forsker udvikler engangsudstyr til at identificere allergierGabriel Caballero Robledo fra Center for Forskning og Avancerede Studier (CINVESTAV) i Monterrey, Mexico, arbejder på design af et lille medicinsk udstyr, der er i stand til at opdage allergier eller
Forsker udvikler engangsudstyr til at identificere allergierGabriel Caballero Robledo fra Center for Forskning og Avancerede Studier (CINVESTAV) i Monterrey, Mexico, arbejder på design af et lille medicinsk udstyr, der er i stand til at opdage allergier eller -
 Polymerforskere sætter aftryk på nanolitografiNanopartikelarrays på en topografisk ujævn overflade. (PhysOrg.com) -- Nanolitografi, eller overflademønstre på nanoskala, er afgørende for moderne teknologi, men er i høj grad udviklet til at møn
Polymerforskere sætter aftryk på nanolitografiNanopartikelarrays på en topografisk ujævn overflade. (PhysOrg.com) -- Nanolitografi, eller overflademønstre på nanoskala, er afgørende for moderne teknologi, men er i høj grad udviklet til at møn
- Renault, Nissan, Mitsubishi bekræfter på ny engagement i alliance
- Undersøgelse:nanostrukturer lover lige så hurtigt, små RRAM-switche
- Ny teknik åbner nye forskningsmuligheder for nye lægemidler og velsmagende mad
- Teaterimprovisationsteknikker viser lovende resultater for naturfagsklassens engagement
- Hvor mange ark papir kan der produceres fra et enkelt træ?
- Dybe rødder af Australiens geologi afsløret


