Ny fotokatalysator kunne muliggøre mere effektiv brintproduktion
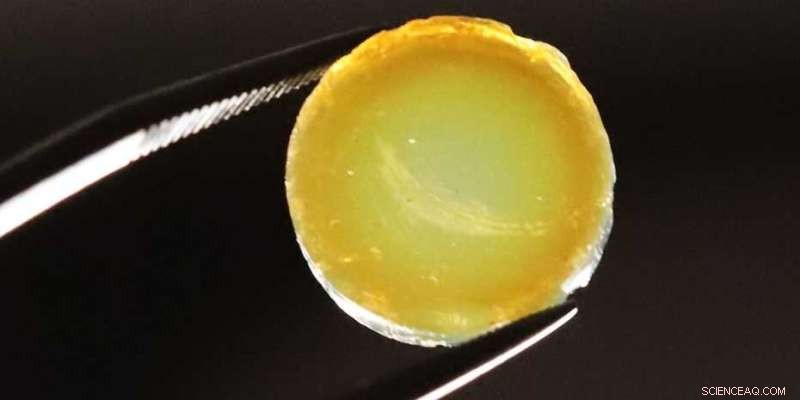
En tabletformet aerogel sammensat af palladium og nitrogen-doterede TiO2 nanopartikler. Kredit:Markus Niederberger / ETH Zürich
Aerogels er ekstraordinære materialer, der har sat Guinness verdensrekorder mere end et dusin gange, blandt andet som verdens letteste faste stoffer.
Professor Markus Niederberger fra Laboratoriet for Multifunktionelle Materialer ved ETH Zürich har arbejdet med disse specielle materialer i nogen tid. Hans laboratorium har specialiseret sig i aerogeler sammensat af krystallinske halvledernanopartikler. "Vi er den eneste gruppe i verden, der kan producere denne slags aerogel i så høj kvalitet," siger han.
En anvendelse for aerogel baseret på nanopartikler er som fotokatalysatorer. Disse anvendes, når en kemisk reaktion skal aktiveres eller accelereres ved hjælp af sollys – et eksempel er produktionen af brint.
Det valgte materiale til fotokatalysatorer er titaniumdioxid (TiO2 ), en halvleder. Men TiO2 har en stor ulempe:den kan kun absorbere UV-delen af sollys - kun omkring 5 procent af spektret. Hvis fotokatalyse skal være effektiv og industrielt anvendelig, skal katalysatoren være i stand til at udnytte et bredere spektrum af bølgelængder.
Udvidelse af spektret med nitrogendoping
Derfor har Niederbergers doktorand Junggou Kwon ledt efter en ny måde at optimere en aerogel lavet af TiO2 nanopartikler. Og hun havde en genial idé:hvis TiO2 nanopartikel aerogel er "doteret" (for at bruge det tekniske udtryk) med nitrogen, således at individuelle oxygenatomer i materialet erstattes af nitrogenatomer, aerogelen kan derefter absorbere yderligere synlige dele af spektret. Dopingprocessen efterlader aerogels porøse struktur intakt. Undersøgelsen om denne metode blev for nylig offentliggjort i tidsskriftet Applied Materials &Interfaces .
Kwon producerede først aerogelen ved hjælp af TiO2 nanopartikler og små mængder af ædelmetallet palladium, som spiller en nøglerolle i den fotokatalytiske produktion af brint. Hun placerede derefter aerogelen i en reaktor og infunderede den med ammoniakgas. Dette fik individuelle nitrogenatomer til at indlejre sig i krystalstrukturen af TiO2 nanopartikler.
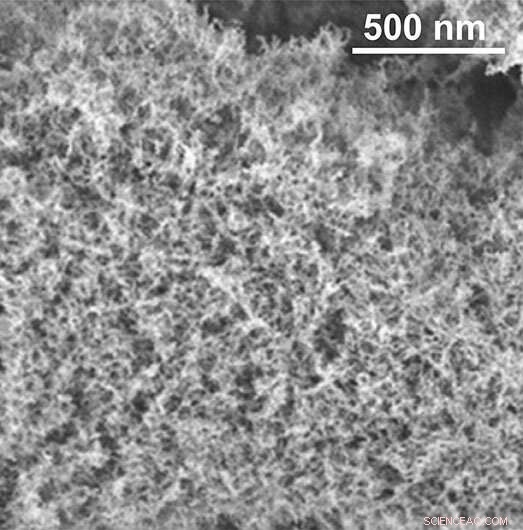
Den svampelignende indre struktur af aerogelen. Kredit:Laboratory for Multifunctional Materials / ETH Zürich
Modificeret aerogel gør reaktionen mere effektiv
For at teste, om en aerogel modificeret på denne måde faktisk øger effektiviteten af en ønsket kemisk reaktion - i dette tilfælde produktionen af brint fra methanol og vand - udviklede Kwon en speciel reaktor, hvori hun direkte placerede aerogelmonolitten. Hun introducerede derefter en damp af vand og methanol til aerogelen i reaktoren, før hun bestrålede den med to LED-lys. The gaseous mixture diffuses through the aerogel's pores, where it is converted into the desired hydrogen on the surface of the TiO2 and palladium nanoparticles.
Kwon stopped the experiment after five days, but up to that point, the reaction was stable and proceeded continuously in the test system. "The process would probably have been stable for longer," Niederberger says. "Especially with regard to industrial applications, it's important for it to be stable for as long as possible." The researchers were satisfied with the reaction's results as well. Adding the noble metal palladium significantly increased the conversion efficiency:using aerogels with palladium produced up to 70 times more hydrogen than using those without.
Increasing the gas flow
This experiment served the researchers primarily as a feasibility study. As a new class of photocatalysts, aerogels offer an exceptional three-dimensional structure and offer potential for many other interesting gas-phase reactions in addition to hydrogen production. Compared to the electrolysis commonly used today, photocatalysts have the advantage that they could be used to produce hydrogen using only light rather than electricity.
Whether the aerogel developed by Niederberger's group will ever be used on a large scale is still uncertain. For example, there is still a question of how to accelerate the gas flow through the aerogel; at the moment, the extremely small pores hinder the gas flow too much. "To operate such a system on an industrial scale, we first have to increase the gas flow and also improve the irradiation of the aerogels," Niederberger says. He and his group are already working on these issues.
Aerogels are exceptional materials. They are extremely light and porous, and boast a huge surface area:one gram of the material can have a surface area of up to 1,200 square meters. Due to their transparency, aerogels have the appearance of "frozen smoke." They are excellent thermal insulators and so are used in aerospace applications and, increasingly, in the thermal insulation of buildings as well. However, their manufacture still requires a huge amount of energy, so the materials are expensive. The first aerogel was produced from silica by the chemist Samuel Kistler in 1931. + Udforsk yderligere
Novel strategy to fabricate 3D-MXene-based electrocatalyst for nitrogen reduction to ammonia
 Varme artikler
Varme artikler
-
 Kan materiale, der konkurrerer med grafen, udvindes fra sten? Ja, hvis...Lag af molybdendisulfid står bedre til at finde applikationer inden for elektronik end grafen. Molybdendisulfid forekommer i naturen som molybdenit, krystallinsk materiale, der ofte har den karakteris
Kan materiale, der konkurrerer med grafen, udvindes fra sten? Ja, hvis...Lag af molybdendisulfid står bedre til at finde applikationer inden for elektronik end grafen. Molybdendisulfid forekommer i naturen som molybdenit, krystallinsk materiale, der ofte har den karakteris -
 Udsortering af nanodiamanter med fluorescerende centreDe optiske kræfter, der virker på nanodiamanten. Nanodiamanten absorberer en del af laserlyset, der skinner på den (Fabs); noget af lyset er også spredt (Fsca). Samspillet mellem disse kræfter forårsa
Udsortering af nanodiamanter med fluorescerende centreDe optiske kræfter, der virker på nanodiamanten. Nanodiamanten absorberer en del af laserlyset, der skinner på den (Fabs); noget af lyset er også spredt (Fsca). Samspillet mellem disse kræfter forårsa -
 En simpel teknik til masseproduktion af ultratynd, højkvalitets molybdæntrioxid nanoarkTransparent fleksibel elektronik baseret på 2D-materialer. Kredit:A*STAR Institute of Materials Research and Engineering Molybdæntrioxid (MoO 3 ) har potentiale som et vigtigt todimensionelt (2-
En simpel teknik til masseproduktion af ultratynd, højkvalitets molybdæntrioxid nanoarkTransparent fleksibel elektronik baseret på 2D-materialer. Kredit:A*STAR Institute of Materials Research and Engineering Molybdæntrioxid (MoO 3 ) har potentiale som et vigtigt todimensionelt (2- -
 Fleksible farveskærme med mikrofluidikDe skematiske principper for enhedsdesign og fremstilling:Den foreslåede mikrofluidiske enhed lavet af polydimethylsiloxan (PDMS) polymer, ved hjælp af standard fotolitografi fremstillingsteknikker ti
Fleksible farveskærme med mikrofluidikDe skematiske principper for enhedsdesign og fremstilling:Den foreslåede mikrofluidiske enhed lavet af polydimethylsiloxan (PDMS) polymer, ved hjælp af standard fotolitografi fremstillingsteknikker ti
- At lære, hvad der får kernen til at krydse
- Fotonik møder overfladevidenskab i en billig og præcis sensor til biologiske væsker
- Simuleringer afslører, hvorfor nogle supernovaeksplosioner producerer så meget mangan og nikkel
- Lille nanomachine gennemfører testkørsel med succes
- Gems of Hawaii
- Tværfaglig forskning tager tid


