En gylden kugle til kræft:Nanopartikler giver en målrettet version af fototermisk behandling mod kræft

Farven på en suspension af nanocages afhænger af tykkelsen af burenes vægge og størrelsen af porer i disse vægge. Ligesom deres farve, deres evne til at absorbere lys og omdanne det til varme kan kontrolleres præcist. Kredit:WUSTL
I et foredrag, han holdt i 1906, den tyske læge Paul Ehrlich opfandt udtrykket Zuberkugel, eller "magisk kugle, "som stenografi for en meget målrettet medicinsk behandling.
Magiske kugler, også kaldet sølvkugler, på grund af den folkloristiske tro på, at kun sølvkugler kan dræbe overnaturlige væsner, fortsat målet for lægemiddeludviklingsindsatsen i dag.
Et team af videnskabsmænd ved Washington University i St. Louis arbejder i øjeblikket på en magisk kugle mod kræft, en sygdom, hvis behandlinger er notorisk vilkårlige og uspecifikke. Men deres kugler er guld frem for sølv. Bogstaveligt talt.
Guldkuglerne er guld nanocages, der, ved indsprøjtning, selektivt akkumuleres i tumorer. Når tumorerne senere bades i laserlys, det omgivende væv er næsten ikke opvarmet, men nanocages omdanner lys til varme, dræber de ondartede celler.
I en artikel netop offentliggjort i tidsskriftet Lille , holdet beskriver den succesrige fototermiske behandling af tumorer i mus.
Teamet omfatter Younan Xia, Ph.D., James M. McKelvey professor i biomedicinsk teknik ved School of Engineering and Applied Science, Michael J. Welch, Ph.D., professor i radiologi og udviklingsbiologi ved School of Medicine, Jingyi Chen, Ph.D., forskningsassistent professor i biomedicinsk teknik og Charles Glaus, Ph.D., en postdoc-forsker ved Radiologisk Institut.
"Vi så betydelige ændringer i tumormetabolisme og histologi, " siger Welch, "hvilket er bemærkelsesværdigt i betragtning af, at arbejdet var undersøgende, laser 'dosis' var ikke blevet maksimeret, og tumorerne var 'passivt' snarere end 'aktivt' målrettet."
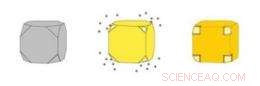
Guld nanocages (højre) er hule kasser lavet ved at udfælde guld på sølv nanokuber (venstre). Sølvet eroderer samtidig inde fra kuben, kommer ind i opløsning gennem porer, der åbner sig i de afklippede hjørner af kuben. Kredit:WUSTL
Hvorfor nanocages bliver varme
Selve nanokagerne er ufarlige. "Guldsalte og guldkolloider er blevet brugt til at behandle gigt i mere end 100 år, " siger Welch. "Folk ved, hvad guld gør i kroppen, og det er inert, så vi håber, at dette vil være en giftfri tilgang."
"Nøglen til fototermisk terapi, " siger Xia, "er burenes evne til effektivt at absorbere lys og omdanne det til varme."
Suspensioner af guld nanocages, som er nogenlunde samme størrelse som en viruspartikel, er ikke altid gule, som man kunne forvente, men i stedet kan være en hvilken som helst farve i regnbuen.
De er farvet af noget, der kaldes en overfladeplasmonresonans. Nogle af elektronerne i guldet er ikke forankret til individuelle atomer, men danner i stedet en fritsvævende elektrongas, Xia forklarer. Lys, der falder på disse elektroner, kan få dem til at svinge som én. Denne kollektive svingning, overflade plasmon, vælger en bestemt bølgelængde, eller farve, ud af det indfaldende lys, og dette bestemmer den farve, vi ser.
Middelalderhåndværkere lavede rubinrødt farvet glas ved at blande guldklorid i smeltet glas, a process that left tiny gold particles suspended in the glass, says Xia.
The resonance — and the color — can be tuned over a wide range of wavelengths by altering the thickness of the cages' walls. For biomedical applications, Xia's lab tunes the cages to 800 nanometers, a wavelength that falls in a window of tissue transparency that lies between 750 and 900 nanometers, in the near-infrared part of the spectrum.
Light in this sweet spot can penetrate as deep as several inches in the body (either from the skin or the interior of the gastrointestinal tract or other organ systems).
The conversion of light to heat arises from the same physical effect as the color. The resonance has two parts. At the resonant frequency, light is typically both scattered off the cages and absorbed by them.
By controlling the cages' size, Xia's lab tailors them to achieve maximum absorption.
Passive targeting
"If we put bare nanoparticles into your body, " says Xia, "proteins would deposit on the particles, and they would be captured by the immune system and dragged out of the bloodstream into the liver or spleen."
For at forhindre dette, the lab coated the nanocages with a layer of PEG, a nontoxic chemical most people have encountered in the form of the laxatives GoLyTELY or MiraLAX. PEG resists the adsorption of proteins, in effect disguising the nanoparticles so that the immune system cannot recognize them.
Instead of being swept from the bloodstream, the disguised particles circulate long enough to accumulate in tumors.
A growing tumor must develop its own blood supply to prevent its core from being starved of oxygen and nutrients. But tumor vessels are as aberrant as tumor cells. They have irregular diameters and abnormal branching patterns, but most importantly, they have thin, leaky walls.
The cells that line a tumor's blood vessel, normally packed so tightly they form a waterproof barrier, are disorganized and irregularly shaped, and there are gaps between them.
The nanocages infiltrate through those gaps efficiently enough that they turn the surface of the normally pinkish tumor black.
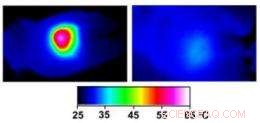
Infrared images made while tumors were irradiated with a laser show that in nanocage-injected mice (left), the surface of the tumor quickly became hot enough to kill cells. In buffer-injected mice (right), the temperature barely budged. This specificity is what makes photothermal therapy so attractive as a cancer therapy. Credit:WUSTL
A trial run
In Welch's lab, mice bearing tumors on both flanks were randomly divided into two groups. The mice in one group were injected with the PEG-coated nanocages and those in the other with buffer solution. Several days later the right tumor of each animal was exposed to a diode laser for 10 minutes.
The team employed several different noninvasive imaging techniques to follow the effects of the therapy. (Welch is head of the oncologic imaging research program at the Siteman Cancer Center of Washington University School of Medicine and Barnes-Jewish Hospital and has worked on imaging agents and techniques for many years.)
During irradiation, thermal images of the mice were made with an infrared camera. As is true of cells in other animals that automatically regulate their body temperature, mouse cells function optimally only if the mouse's body temperature remains between 36.5 and 37.5 degrees Celsius (98 to 101 degrees Fahrenheit).
At temperatures above 42 degrees Celsius (107 degrees Fahrenheit) the cells begin to die as the proteins whose proper functioning maintains them begin to unfold.
In the nanocage-injected mice, the skin surface temperature increased rapidly from 32 degrees Celsius to 54 degrees C (129 degrees F).
In the buffer-injected mice, imidlertid, the surface temperature remained below 37 degrees Celsius (98.6 degrees Fahrenheit).
To see what effect this heating had on the tumors, the mice were injected with a radioactive tracer incorporated in a molecule similar to glucose, the main energy source in the body. Positron emission and computerized tomography (PET and CT) scans were used to record the concentration of the glucose lookalike in body tissues; the higher the glucose uptake, the greater the metabolic activity.
The tumors of nanocage-injected mice were significantly fainter on the PET scans than those of buffer-injected mice, indicating that many tumor cells were no longer functioning.
The tumors in the nanocage-treated mice were later found to have marked histological signs of cellular damage.
Active targeting
The scientists have just received a five-year, $2, 129, 873 grant from the National Cancer Institute to continue their work with photothermal therapy.
Despite their results, Xia is dissatisfied with passive targeting. Although the tumors took up enough gold nanocages to give them a black cast, only 6 percent of the injected particles accumulated at the tumor site.
Xia would like that number to be closer to 40 percent so that fewer particles would have to be injected. He plans to attach tailor-made ligands to the nanocages that recognize and lock onto receptors on the surface of the tumor cells.
In addition to designing nanocages that actively target the tumor cells, the team is considering loading the hollow particles with a cancer-fighting drug, so that the tumor would be attacked on two fronts.
But the important achievement, from the point of view of cancer patients, is that any nanocage treatment would be narrowly targeted and thus avoid the side effects patients dread.
The TV and radio character the Lone Ranger used only silver bullets, allegedly to remind himself that life was precious and not to be lightly thrown away. If he still rode today, he might consider swapping silver for gold.
 Varme artikler
Varme artikler
-
 Løftet og faren ved nanoteknologiComputer-gengivet billede inde i et kulstof nanorør. Kredit:Geoff Hutchison Forskere ved Northwestern University har fundet en måde at opdage metastatisk brystkræft ved at arrangere DNA-strenge i
Løftet og faren ved nanoteknologiComputer-gengivet billede inde i et kulstof nanorør. Kredit:Geoff Hutchison Forskere ved Northwestern University har fundet en måde at opdage metastatisk brystkræft ved at arrangere DNA-strenge i -
 Udnyttelse af mikroorganismer til smarte mikrosystemerIntegration af en struktur med Vorticella (Øverst, EN, B). Gentagne bevægelser af en struktur på grund af kraften fra Vorticella og flow (nedre, C, D). Kredit:Toyohashi University of Technology.
Udnyttelse af mikroorganismer til smarte mikrosystemerIntegration af en struktur med Vorticella (Øverst, EN, B). Gentagne bevægelser af en struktur på grund af kraften fra Vorticella og flow (nedre, C, D). Kredit:Toyohashi University of Technology. -
 Nyt glasstempel kan gøre det billigere, mere præcise biosensorerEt glasstempel gengiver præcist, raderinger i sølv i nanometerskala. Den originale gravering, afbilledet ovenfor, er 10 mikron bred - mindre end en fjerdedel af diameteren af et menneskehår. Billede
Nyt glasstempel kan gøre det billigere, mere præcise biosensorerEt glasstempel gengiver præcist, raderinger i sølv i nanometerskala. Den originale gravering, afbilledet ovenfor, er 10 mikron bred - mindre end en fjerdedel af diameteren af et menneskehår. Billede -
 Udforskning af katalytiske reaktioner på nanoskalaSkematisk over TERS-apparatet og den undersøgte katalytiske reaktion National Physical Laboratory (NPL) har brugt en ny billeddannelsesfunktion - tip-forstærket Raman-spektroskopi - til at kortlæg
Udforskning af katalytiske reaktioner på nanoskalaSkematisk over TERS-apparatet og den undersøgte katalytiske reaktion National Physical Laboratory (NPL) har brugt en ny billeddannelsesfunktion - tip-forstærket Raman-spektroskopi - til at kortlæg
- Undersøgelse:Børn er interesserede i politik, men har brug for bedre uddannelse fra forældre og s…
- Sådan Remagnetiserer du et Kompass Needle
- Hvorfor du skal blande dig i geoingeniørdebatten - nu
- Er der et glasloft i akademisk udgivelse?
- Skabte Apollo -missionerne opvarmning på månen?
- Sådan fungerer vejrballoner


