Nobel i kemi ærer en grønnere måde at bygge molekyler på

Göran K Hansson, Permanent sekretær for Det Kongelige Svenske Videnskabsakademi, centrum, annoncerer vinderne af 2021 Nobelprisen i kemi, i Stockholm, Sverige, Onsdag, 6. okt. 2021. Professor Pernilla Wittung-Stafhede, sidder til venstre og professor Peter Somfai til højre. To videnskabsmænd har vundet Nobelprisen i kemi for at finde en "genial" ny måde at bygge molekyler på, som kan bruges til at lave alt fra medicin til smagsstoffer til fødevarer. Benjamin List over Tyskland og Skotland-fødte David W.C. MacMillan udviklede "asymmetrisk organokatalyse." Goran Hansson fra Det Kongelige Svenske Videnskabsakademi sagde onsdag, at arbejdet allerede har haft en betydelig indvirkning på farmaceutisk forskning. Kredit:Claudio Bresciani/TT New Agency via AP
To videnskabsmænd vandt onsdag Nobelprisen i kemi for at finde en genial og miljømæssigt renere måde at bygge molekyler på - en tilgang, der nu bruges til at fremstille en række forskellige forbindelser, herunder medicin og pesticider.
Arbejdet af Benjamin List og David W.C. MacMillan har givet videnskabsfolk mulighed for at producere disse molekyler billigere, effektivt, sikkert og med væsentligt mindre farligt affald.
"Det gavner allerede menneskeheden meget, sagde Pernilla Wittung-Stafshede, medlem af Nobelpanelet.
Det var anden dag i træk, at en Nobel belønnede arbejde, der havde miljømæssige konsekvenser. Fysikprisen hædrede udviklinger, der udvidede vores forståelse af klimaændringer, blot uger før starten af globale klimaforhandlinger i Skotland.
Kemiprisen fokuserede på fremstilling af molekyler. Det kræver at forbinde atomer sammen i specifikke arrangementer, en ofte svær og langsom opgave. Indtil begyndelsen af årtusindet, kemikere havde kun to metoder - eller katalysatorer - til at fremskynde processen, ved brug af enten komplicerede enzymer eller metalkatalysatorer.
Det hele ændrede sig, da List, fra Max Planck Instituttet i Tyskland, og MacMillan, fra Princeton University i New Jersey, uafhængigt rapporteret, at små organiske molekyler kan bruges til at udføre arbejdet. De nye værktøjer har været vigtige for at udvikle medicin og minimere fejl i fremstillingen af lægemidler, herunder problemer, der kan forårsage skadelige bivirkninger.
Johan Åqvist, formand for Nobelpanelet, kaldte metoden som "simpel som den er genial."
"Faktum er, at mange mennesker har undret sig over, hvorfor vi ikke tænkte på det tidligere, " han tilføjede.
MacMillan sagde, at at vinde prisen gjorde ham "forbløffet, chokeret, lykkelig, meget stolt."
"Jeg voksede op i Skotland, et barn fra arbejderklassen. Min far er stålarbejder. Min mor var hjemmehjælper. … Jeg var så heldig at få en chance for at komme til Amerika, at lave min ph.d., " han sagde.
Faktisk, sagde han på en pressekonference i Princeton, han planlagde at følge sin ældre bror ind i fysikken, men fysiktimerne på college var kl. 8.00 i et koldt og utæt klasseværelse i det regnfulde Skotland, mens kemikurserne var to timer senere i varmere, tørre rum. Da han fortalte den historie, han sagde, at han kunne høre sin kone trygle ham om ikke at dele det.
-

David W.C. MacMillan, en af to vindere af Nobelprisen i kemi, smiler, da han bliver interviewet uden for Frick Chemistry Laboratory og Department of Chemistry på Princeton University, Onsdag, 6. okt. 2021, i Princeton, N.J. Arbejdet af Benjamin List of Germany og Skotland-fødte David W.C. MacMillan blev præmieret for at finde en "genial" og miljømæssigt renere måde at bygge molekyler på, der kan bruges til at lave alt fra medicin til smagsstoffer til fødevarer. Kredit:AP Photo/John Minchillo
-

Dette udaterede billede blev leveret onsdag, 6. okt. 2021 af det tyske Max-Plank-Society viser den tyske videnskabsmand Benjamin List. To videnskabsmænd har vundet Nobelprisen i kemi for at finde en "genial" ny måde at bygge molekyler på, som kan bruges til at lave alt fra medicin til smagsstoffer til fødevarer. Benjamin List over Tyskland og Skotland-fødte David W.C. MacMillan udviklede "asymmetrisk organokatalyse." Kredit:David Ausserhofer, Max-Plank-Society via AP
Hans sagde, at inspirationen til hans nobelvindende arbejde kom, da han tænkte på den beskidte proces med at fremstille kemikalier - en, der kræver forholdsregler, han sammenlignede med dem, der blev taget på atomkraftværker.
Hvis han kunne udtænke en måde at lave medicin hurtigere på på helt andre måder, der ikke krævede kar med metalkatalysatorer, processen ville være mere sikker for både arbejdere og planeten, ræsonnerede han.
List sagde, at han oprindeligt ikke vidste, at MacMillan arbejdede med det samme emne, og regnede med, at hans egen anelse måske bare var en "dum idé" - indtil det virkede. I det eureka-øjeblik, "Jeg følte, at det her kunne være noget stort, " sagde den 53-årige.
H.N. Cheng, præsident for American Chemical Society, sagde, at prismodtagerne udviklede "nye tryllestave."
Før deres arbejde, "de almindeligt anvendte standardkatalysatorer var metaller, som ofte har miljømæssige ulemper, " sagde Cheng. "De samler sig, de udvasker, de kan være farlige."

Dette udaterede billede blev leveret onsdag, 6. okt. 2021 af det tyske Max-Plank-Society viser den tyske videnskabsmand Benjamin List, centrum. To videnskabsmænd har vundet Nobelprisen i kemi for at finde en "genial" ny måde at bygge molekyler på, som kan bruges til at lave alt fra medicin til smagsstoffer til fødevarer. Benjamin List over Tyskland og Skotland-fødte David W.C. MacMillan udviklede "asymmetrisk organokatalyse." Kredit:David Ausserhofer, Max-Plank-Society via AP
De katalysatorer, som MacMillan og List var banebrydende for "er økologiske, så de nedbrydes hurtigere, og de er også billigere, " han sagde.
Nobelpanelet bemærkede, at deres bidrag gjorde produktionen af vigtige lægemidler lettere, herunder en antiviral og en anti-angst medicin.
"En måde at se deres arbejde på er som molekylært tømrerarbejde, " sagde John Lorsch, direktør for National Institute of General Medical Sciences ved U.S. National Institutes of Health.
"De har fundet måder at ikke kun fremskynde den kemiske sammenføjning, " han sagde, "men for at sikre, at det kun går i enten højre- eller venstrehånds retning."
Evnen til at kontrollere den orientering, hvori nye atomer tilføjes til molekyler, er vigtig. Undladelse af at gøre det kan resultere i bivirkninger i medicin, Nobelpanelet forklarede, citerer det katastrofale eksempel på thalidomid, som forårsagede alvorlige fødselsdefekter hos børn.
-

David W.C. MacMillan, en af to vindere af Nobelprisen i kemi, er interviewet uden for Frick Chemistry Laboratory og Department of Chemistry ved Princeton University, Onsdag, 6. okt. 2021, i Princeton, N.J. Arbejdet af Benjamin List of Germany og Skotland-fødte David W.C. MacMillan blev præmieret for at finde en "genial" og miljømæssigt renere måde at bygge molekyler på, som kan bruges til at lave alt fra medicin til smagsstoffer til fødevarer. Kredit:AP Photo/John Minchillo
-

David W.C. MacMillan, en af to vindere af Nobelprisen i kemi, smiler, da han bliver interviewet uden for Frick Chemistry Laboratory og Department of Chemistry på Princeton University, Onsdag, 6. okt. 2021, i Princeton, N.J. Arbejdet af Benjamin List of Germany og Skotland-fødte David W.C. MacMillan blev præmieret for at finde en "genial" og miljømæssigt renere måde at bygge molekyler på, der kan bruges til at lave alt fra medicin til smagsstoffer til fødevarer. Kredit:AP Photo/John Minchillo
-

Den tyske videnskabsmand Benjamin List vinker ud af en bil, da han ankommer til Max-Planck-instituttet for kulforskning i Muelheim, Tyskland, Onsdag, 6. okt. 2021. To videnskabsmænd har vundet Nobelprisen i kemi for at finde en "genial" ny måde at bygge molekyler på, som kan bruges til at lave alt fra medicin til smagsstoffer til fødevarer. Benjamin List over Tyskland og Skotland-fødte David W.C. MacMillan udviklede "asymmetrisk organokatalyse." Kredit:AP Photo/Martin Meissner
-

Den tyske videnskabsmand Benjamin List poserer ved siden af en plakat med en medalje af Alfred Nobel, da han ankommer til Max-Planck-instituttet for kulforskning i Muelheim, Tyskland, Onsdag, 6. okt. 2021. To videnskabsmænd har vundet Nobelprisen i kemi for at finde en "genial" ny måde at bygge molekyler på, som kan bruges til at lave alt fra medicin til smagsstoffer til fødevarer. Benjamin List over Tyskland og Skotland-fødte David W.C. MacMillan udviklede "asymmetrisk organokatalyse." Kredit:AP Photo/Martin Meissner
-

Den tyske videnskabsmand Benjamin List drikker champagne, da han ankommer til Max-Planck-instituttet for kulforskning i Muelheim, Tyskland, Onsdag, 6. okt. 2021. To videnskabsmænd har vundet Nobelprisen i kemi for at finde en "genial" ny måde at bygge molekyler på, som kan bruges til at lave alt fra medicin til smagsstoffer til fødevarer. Benjamin List over Tyskland og Skotland-fødte David W.C. MacMillan udviklede "asymmetrisk organokatalyse." Kredit:AP Photo/Martin Meissner
-

Den tyske videnskabsmand Benjamin List ankommer til Max-Planck-instituttet for kulforskning i Muelheim, Tyskland, Onsdag, 6. okt. 2021, efter at han blev informeret om at vinde Nobelprisen i kemi. To videnskabsmænd har vundet Nobelprisen i kemi for at finde en "genial" ny måde at bygge molekyler på, som kan bruges til at lave alt fra medicin til smagsstoffer til fødevarer. Benjamin List over Tyskland og Skotland-fødte David W.C. MacMillan udviklede "asymmetrisk organokatalyse." Kredit:AP Photo/Martin Meissner
Siden forskernes opdagelse, værktøjet er blevet yderligere forfinet, gør det mange gange mere effektivt.
Peter Somfai, et andet medlem af udvalget, understregede vigtigheden af opdagelsen for verdensøkonomien.
"Det er blevet anslået, at katalyse er ansvarlig for omkring 35% af verdens BNP, hvilket er en ret imponerende figur, " sagde han. "Hvis vi har et mere miljøvenligt alternativ, det forventes, at det vil gøre en forskel."
NIH støttede Lists forskning med en bevilling i 2002. MacMillans arbejde har modtaget støtte fra NIH siden 2000, for i alt omkring 14,5 millioner dollars til dato.
"Det er et godt eksempel på at støtte grundlæggende videnskab, at du ikke nødvendigvis ved, hvor det skal hen", men det kan have stor indflydelse, sagde Francis Collins, NIH direktør.
Nobelprisen kommer med en guldmedalje og 10 millioner svenske kroner, , eller mere end 1,14 millioner dollars. Pengene kommer fra et legat efterladt af præmiens skaber, Den svenske opfinder Alfred Nobel, der døde i 1895.
I løbet af de kommende dage, Nobelprisen vil blive uddelt i litteratur, fred og økonomi.
*****
Nobelkomiteens pressemeddelelse:Nobelprisen i kemi 2021
Det Kongelige Svenske Videnskabsakademi har besluttet at tildele Nobelprisen i kemi 2021 til
Benjamin Liste
Max-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr, Tyskland
David W.C. MacMillan
Princeton University, USA
"til udvikling af asymmetrisk organokatalyse"
Et genialt værktøj til at bygge molekyler
At bygge molekyler er en svær kunst. Benjamin List og David MacMillan tildeles Nobelprisen i kemi 2021 for deres udvikling af et præcist nyt værktøj til molekylær konstruktion:organokatalyse. Dette har haft stor indflydelse på den farmaceutiske forskning, og har gjort kemien grønnere.
Mange forskningsområder og industrier er afhængige af kemikeres evne til at konstruere molekyler, der kan danne elastiske og holdbare materialer, lagre energi i batterier eller hæmme udviklingen af sygdomme. Dette arbejde kræver katalysatorer, som er stoffer, der styrer og fremskynder kemiske reaktioner, uden at blive en del af det endelige produkt. For eksempel, katalysatorer i biler omdanner giftige stoffer i udstødningsgasser til harmløse molekyler. Vores kroppe indeholder også tusindvis af katalysatorer i form af enzymer, som mejsler de molekyler, der er nødvendige for livet.
Katalysatorer er således grundlæggende værktøjer for kemikere, men forskere har længe troet, at der var, i princippet, kun to typer katalysatorer tilgængelige:metaller og enzymer. Benjamin List og David MacMillan tildeles Nobelprisen i kemi 2021, fordi de i 2000, uafhængige af hinanden, udviklet en tredje type katalyse. Det kaldes asymmetrisk organokatalyse og bygger på små organiske molekyler.
"Dette koncept for katalyse er lige så enkelt, som det er genialt, og faktum er, at mange mennesker har undret sig over, hvorfor vi ikke tænkte på det tidligere, siger Johan Åqvist, som er formand for Nobelkomiteen for Kemi.
Organiske katalysatorer har en stabil ramme af kulstofatomer, hvortil mere aktive kemiske grupper kan knytte sig. Disse indeholder ofte almindelige elementer såsom ilt, nitrogen, svovl eller fosfor. Det betyder, at disse katalysatorer er både miljøvenlige og billige at producere.
Den hurtige ekspansion i brugen af organiske katalysatorer skyldes primært deres evne til at drive asymmetrisk katalyse. Når molekyler bygges, Der opstår ofte situationer, hvor to forskellige molekyler kan dannes, der – ligesom vores hænder – er hinandens spejlbillede. Kemikere vil ofte kun have én af disse, især ved fremstilling af lægemidler.
Organokatalyse har udviklet sig med en forbløffende hastighed siden 2000. Benjamin List og David MacMillan er fortsat førende på området, og har vist, at organiske katalysatorer kan bruges til at drive mange kemiske reaktioner. Ved at bruge disse reaktioner, forskere kan nu mere effektivt konstruere alt fra nye lægemidler til molekyler, der kan fange lys i solceller. På denne måde organokatalysatorer bringer den største fordel for menneskeheden.
Populær information
Deres værktøjer revolutionerede konstruktionen af molekyler
Kemikere kan skabe nye molekyler ved at forbinde små kemiske byggesten, men det er svært at kontrollere usynlige stoffer, så de binder på den ønskede måde. Benjamin List og David MacMillan tildeles Nobelprisen i kemi 2021 for deres udvikling af et nyt og genialt værktøj til molekyleopbygning:organokatalyse. Dets anvendelser omfatter forskning i nye lægemidler, og det har også bidraget til at gøre kemi grønnere.
Mange industrier og forskningsområder er afhængige af kemikeres evne til at bygge nye og funktionelle molekyler. Det kan være alt fra stoffer, der fanger lys i solceller eller lagrer energi i batterier, til molekyler, der kan lave lette løbesko eller hæmme sygdomsforløbet i kroppen.
Imidlertid, hvis vi sammenligner naturens evne til at bygge kemiske kreationer med vores egne, vi sad længe fast i stenalderen. Evolution har produceret utroligt specifikke værktøjer, enzymer, til at konstruere de molekylære komplekser, der giver livet dets former, farver og funktioner. I første omgang, da kemikere isolerede disse kemiske mesterværker, de så bare på dem med beundring. Hammerne og mejslerne i deres egne værktøjskasser til molekylær konstruktion var stumpe og upålidelige, så de endte ofte med masser af uønskede biprodukter, når de kopierede naturens produkter.
Nye værktøjer til finere kemi
Hvert nyt værktøj, som kemikere har tilføjet deres værktøjskasse, har øget præcisionen af deres molekylære konstruktioner. Langsomt men sikkert, kemien er gået fra mejsling i sten til noget mere som fint håndværk. Dette har været til stor gavn for menneskeheden, og flere af disse værktøjer er blevet belønnet med Nobelprisen i kemi.
Opdagelsen, der tildeles Nobelprisen i kemi 2021, har taget molekylær konstruktion til et helt nyt niveau. Det har ikke kun gjort kemi grønnere, men gjorde det også meget nemmere at producere asymmetriske molekyler. Under kemisk konstruktion opstår der ofte en situation, hvor to molekyler kan dannes, der – ligesom vores hænder – er hinandens spejlbillede. Kemikere vil ofte bare have et af disse spejlbilleder, især ved fremstilling af lægemidler, men det har været svært at finde effektive metoder til at gøre dette. The concept developed by Benjamin List and David MacMillan – asymmetric organocatalysis – is as simple as it is brilliant. The fact is that many people have wondered why we didn't think of it earlier.
Why indeed? This is no easy question to answer, but before we even try we need to take a quick look back at history. We will define the terms catalysis and catalyst, and set the stage for the Nobel Prize in Chemistry 2021.
Catalysts accelerate chemical reactions
In the nineteenth century, when chemists began exploring the ways that different chemicals react with each other, they made some strange discoveries. For eksempel, if they put silver in a beaker with hydrogen peroxide (H2O2), the hydrogen peroxide suddenly began to break down into water (H2O) and oxygen (O2). But the silver – which started the process – did not seem affected by the reaction at all. Tilsvarende a substance obtained from sprouting grains could break down starch into glucose.
In 1835, the renowned Swedish chemist Jacob Berzelius started to see a pattern in this. In the Royal Swedish Academy of Sciences' annual report, describing the latest progress in physics and chemistry, he writes about a new "force" that can "generate chemical activity". He listed several examples in which just the presence of a substance started a chemical reaction, stating how this phenomenon appeared to be considerably more common than was previously thought. He believed that the substance had a catalytic force and called the phenomenon itself catalysis.
Catalysts produce plastic, perfume and flavoursome food
A great deal of water has run through chemists' pipettes since Berzelius' time. They have discovered a multitude of catalysts that can break down molecules or join them together. Thanks to these, they can now carve out the thousands of different substances we use in our everyday lives, such as pharmaceuticals, plastics, perfumes and food flavourings. The fact is, it is estimated that 35 per cent of the world's total GDP in some way involves chemical catalysis.
In principle, all catalysts discovered before the year 2000 belonged to one of two groups:they were either metals or enzymes. Metals are often excellent catalysts because they have a special ability to temporarily accommodate electrons or to provide them to other molecules during a chemical process. This helps loosen the bonds between the atoms in a molecule, so bonds that are otherwise strong can be broken and new ones can form.
Imidlertid, one problem with some metal catalysts is that they are very sensitive to oxygen and water so, for these to work, they need an environment free of oxygen and moisture. This is difficult to achieve in large-scale industries. Også, many metal catalysts are heavy metals, which can be harmful to the environment.
Life's catalysts work with astounding precision
The second form of catalyst is comprised of the proteins known as enzymes. All living things have thousands of different enzymes that drive the chemical reactions necessary for life. Many enzymes are specialists in asymmetric catalysis and, i princippet, always form one mirror image out of the two that are possible. They also work side by side; when one enzyme is finished with a reaction, another one takes over. På denne måde they can build complicated molecules with amazing precision, such as cholesterol, chlorophyll or the toxin called strychnine, which is one of the most complex molecules we know of (we will return to this).
Because enzymes are such efficient catalysts, researchers in the 1990s tried to develop new enzyme variants to drive the chemical reactions needed by humanity. One research group working on this was based at the Scripps Research Institute in southern California and was led by the late Carlos F. Barbas III. Benjamin List had a postdoctoral position in Barbas' research group when the brilliant idea that led to one of the discoveries behind this year's Nobel Prize in Chemistry was born.
Benjamin List thinks outside the box…
Benjamin List worked with catalytic antibodies. Normally, antibodies attach to foreign viruses or bacteria in our bodies, but the researchers at Scripps redesigned them so they could drive chemical reactions instead.
During his work with catalytic antibodies, Benjamin List started to think about how enzymes actually work. They are usually huge molecules that are built from hundreds of amino acids. In addition to these amino acids, a significant proportion of enzymes also have metals that help drive chemical processes. But – and this is the point – many enzymes catalyse chemical reactions without the help of metals. I stedet, the reactions are driven by one or a few individual amino acids in the enzyme. Benjamin List's out-of-the-box question was:do amino acids have to be part of an enzyme in order to catalyse a chemical reaction? Or could a single amino acid, or other similar simple molecules, do the same job?
…with a revolutionary result
He knew that there was research from the early 1970s where an amino acid called proline had been used as a catalyst – but that was more than 25 years ago. Surely, if proline really had been an effective catalyst, someone would have continued working on it?
This is more or less what Benjamin List thought; he assumed that the reason why no one had continued studying the phenomenon was that it had not worked particularly well. Without any real expectations, he tested whether proline could catalyse an aldol reaction, in which carbon atoms from two different molecules are bonded together. It was a simple attempt that, amazingly, worked straight away.
Benjamin List staked out his future
With his experiments, Benjamin List not only demonstrated that proline is an efficient catalyst, but also that this amino acid can drive asymmetric catalysis. Of the two possible mirror images, it was much more common for one of them to form than the other.
Unlike the researchers who had previously tested proline as a catalyst, Benjamin List understood the enormous potential it could have. Compared to both metals and enzymes, proline is a dream tool for chemists. It is a very simple, cheap and environmentally-friendly molecule. When he published his discovery in February 2000, List described asymmetric catalysis with organic molecules as a new concept with many opportunities:"The design and screening of these catalysts is one of our future aims".
Imidlertid, he was not alone in this. In a laboratory further north in California, David MacMillan was also working towards the same goal.
David MacMillan leaves sensitive metals behind…
Two years previously, David MacMillan had moved from Harvard to UC Berkeley. At Harvard he had worked on improving asymmetric catalysis using metals. This was an area which was attracting a lot of attention from researchers, but David MacMillan noted how the catalysts that were developed were rarely used in industry. He started to think about why, and assumed that the sensitive metals were quite simply too difficult and expensive to use. Achieving the oxygen-free and moisturefree conditions demanded by some metal catalysts is relatively simple in a laboratory, but conducting large-scale industrial manufacturing in such conditions is complicated.
His conclusion was that if the chemical tools he was developing were to be useful, he needed a rethink. Så, when he moved to Berkeley, he left the metals behind.
…and develops a simpler form of catalyst
I stedet, David MacMillan started to design simple organic molecules which – just like metals – could temporarily provide or accommodate electrons. Her, we need to define what organic molecules are – in brief, these are the molecules that build all living things. They have a stable framework of carbon atoms. Active chemical groups are attached to this carbon framework, and they often contain oxygen, nitrogen, sulphur or phosphorus.
Organic molecules thus consist of simple and common elements but, depending on how they are put together, they can have complex properties. David MacMillan's knowledge of chemistry told him that for an organic molecule to catalyse the reaction he was interested in, it needed to be able to form an iminium ion. This contains a nitrogen atom, which has an inherent affinity for electrons.
He selected several organic molecules with the right properties, and then tested their ability to drive a Diels–Alder reaction, which chemists use to build rings of carbon atoms. Just as he had hoped and believed, it worked brilliantly. Some of the organic molecules were also excellent at asymmetric catalysis. Of two possible mirror images, one of them comprised more than 90 per cent of the product.
David MacMillan coins the term organocatalysis
When David MacMillan was ready to publish his results, he realised that the concept for catalysis he had discovered needed a name. The fact was that researchers had previously succeeded in catalysing chemical reactions using small organic molecules, but these were isolated examples and no one had realised that the method could be generalised.
David MacMillan wanted to find a term to describe the method so other researchers would understand that there were more organic catalysts to discover. His choice was organocatalysis.
In January 2000, just before Benjamin List published his discovery, David MacMillan submitted his manuscript for publication in a scientific journal. The introduction states:"Herein, we introduce a new strategy for organocatalysis that we expect will be amenable to a range of asymmetric transformations".
The use of organocatalysis has boomed
Independently of each other, Benjamin List and David MacMillan had discovered an entirely new concept for catalysis. Since 2000, developments in this area can almost be likened to a gold rush, in which List and MacMillan maintain leading positions. They have designed multitudes of cheap and stable organocatalysts, which can be used to drive a huge variety of chemical reactions.
Not only do organocatalysts often consist of simple molecules, in some cases – just like nature's enzymes – they can work on a conveyor belt. Tidligere, in chemical production processes it was necessary to isolate and purify each intermediate product, otherwise the volume of byproducts would be too great. This led to some of the substance being lost at every step of a chemical construction.
Organocatalysts are much more forgiving as, relatively often, several steps in a production process can be performed in an unbroken sequence. This is called a cascade reaction, which can considerably reduce waste in chemical manufacturing.
Strychnine synthesis now 7, 000 times more efficient
One example of how organocatalysis has led to more efficient molecular constructions is the synthesis of the natural, and astoundingly complex, strychnine molecule. Many people will recognise strychnine from books by Agatha Christie, queen of the murder mystery. Imidlertid, for chemists, strychnine is like a Rubik's Cube:a challenge that you want to solve in as few steps as possible.
When strychnine was first synthesised, in 1952, it required 29 different chemical reactions and only 0.0009 per cent of the initial material formed strychnine. The rest was wasted.
I 2011 researchers were able to use organocatalysis and a cascade reaction to build strychnine in just 12 steps, and the production process was 7, 000 times more efficient.
Organocatalysis is most important in pharmaceutical production
Organocatalysis has had a significant impact on pharmaceutical research, which frequently requires asymmetric catalysis. Until chemists could conduct asymmetric catalysis, many pharmaceuticals contained both mirror images of a molecule; one of these was active, while the other could sometimes have unwanted effects. A catastrophic example of this was the thalidomide scandal in the 1960s, in which one mirror image of the thalidomide pharmaceutical caused serious deformities in thousands of developing human embryos.
Using organocatalysis, researchers can now make large volumes of different asymmetric molecules relatively simply. For eksempel, they can artificially produce potentially curative substances that can otherwise only be isolated in small amounts from rare plants or deep-sea organisms.
At pharmaceutical companies, the method is also used to streamline the production of existing pharmaceuticals. Examples of this include paroxetine, which is used to treat anxiety and depression, and the antiviral medication oseltamivir, which is used to treat respiratory infections.
Simple ideas are often the most difficult to imagine
It is possible to list thousands of examples of how organocatalysis is used – but why did no one come up with this simple, green and cheap concept for asymmetric catalysis earlier? This question has many answers. One is that the simple ideas are often the most difficult to imagine. Our view is obscured by strong preconceptions about how the world should work, such as the idea that only metals or enzymes can drive chemical reactions. Benjamin List and David MacMillan succeeded in seeing past these preconceptions to find an ingenious solution to a problem with which chemists had struggled for decades. Organocatalysts are thus bringing – right now – the greatest benefit to humankind.
© 2021 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed without permission.
 Varme artikler
Varme artikler
-
 Ny platform til at skabe og karakterisere materialeblandinger kan fremskynde udviklingen markantYale University ph.d.-studerende Kristof Toth (billedet ovenfor) med det elektrosprayaflejringsværktøj, han designede, bygget, og valideret i samarbejde med stabsforsker Gregory Doerk fra Brookhaven L
Ny platform til at skabe og karakterisere materialeblandinger kan fremskynde udviklingen markantYale University ph.d.-studerende Kristof Toth (billedet ovenfor) med det elektrosprayaflejringsværktøj, han designede, bygget, og valideret i samarbejde med stabsforsker Gregory Doerk fra Brookhaven L -
 Ny metode opdager hurtigt spormængder af små molekyle forbindelserKredit:@Lion_on_helium/MIPT Russiske forskere fra Prokhorov General Physics Institute of the Russian Academy of Sciences (GPI RAS) og Moskva Institute of Physics and Technology (MIPT) har udviklet
Ny metode opdager hurtigt spormængder af små molekyle forbindelserKredit:@Lion_on_helium/MIPT Russiske forskere fra Prokhorov General Physics Institute of the Russian Academy of Sciences (GPI RAS) og Moskva Institute of Physics and Technology (MIPT) har udviklet -
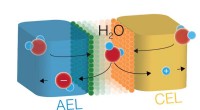 Forskere adskiller vand effektivt med nye katalysatorerForskning i et kemilaboratorium fra University of Oregon har fremskyndet effektiviteten af den katalytiske vanddissociationsreaktion i bipolære membraner. Et team på tre medlemmer brugte en membran-
Forskere adskiller vand effektivt med nye katalysatorerForskning i et kemilaboratorium fra University of Oregon har fremskyndet effektiviteten af den katalytiske vanddissociationsreaktion i bipolære membraner. Et team på tre medlemmer brugte en membran- -
 Udnyttelse af solen til at bringe frisk vand til fjerntliggende eller katastroferamte samfundKredit:CC0 Public Domain En enhed, der tager en ny tilgang til at fjerne salt fra vand, er blevet udviklet i Bath, baner vejen for små, solcelledrevne afsaltningsenheder Forskere ved University o
Udnyttelse af solen til at bringe frisk vand til fjerntliggende eller katastroferamte samfundKredit:CC0 Public Domain En enhed, der tager en ny tilgang til at fjerne salt fra vand, er blevet udviklet i Bath, baner vejen for små, solcelledrevne afsaltningsenheder Forskere ved University o
- Hvordan man beskytter hjem mod naturbrande er emnet i professorernes nye bog
- Forklaring af tyngdekraft uden strengteori
- Den russiske teknologigigant gør håb om en smartphone
- Hvad slags vejr virker Nimbostratus Clouds?
- Hvordan Asteroid Mining vil fungere
- Fritstående anode til brug for at hjælpe mikrobielle brændselsceller med at omdanne affald til el…


