How it is different a.m.u of carbon isotopes?
What are Isotopes?
* Isotopes are atoms of the same element (like carbon) that have the same number of protons but a different number of neutrons.
* Da antallet af protoner definerer elementet, opfører isotoper sig kemisk næsten identisk, men har lidt forskellige masser på grund af det varierende antal neutroner.
Carbon Isotopes
Carbon has two main naturally occurring isotopes:
1. Carbon-12 (¹²C): This is the most common isotope, making up about 98.9% of all carbon found on Earth. It has 6 protons and 6 neutrons. Its atomic mass is exactly 12 amu by definition.
2. Carbon-13 (¹³C): This isotope is less abundant, making up about 1.1% of carbon. It has 6 protons and 7 neutrons. Its atomic mass is approximately 13 amu .
Key Points
* Definition of amu: The atomic mass unit (amu) is defined as 1/12th the mass of a single carbon-12 atom.
* Mass Difference: The difference in amu between carbon isotopes is primarily due to the extra neutron in ¹³C compared to ¹²C. This difference is relatively small but measurable.
Why Does the Difference Matter?
* Radioactive Isotopes: Some isotopes of carbon, like Carbon-14 (¹⁴C), are radioactive and have applications in dating archaeological artifacts.
* massespektrometri: Instrumenter som massespektrometre kan skelne mellem isotoper baseret på deres masse, hjælpe forskere med at studere deres relative overflod og bruge dem i forskellige anvendelser.
Lad mig vide, om du vil have flere detaljer om en bestemt kulstofisotop eller dens applikationer!
Sidste artikelHvordan bliver vand til et fast stof?
Næste artikelHvad er fem metalfinish?
 Varme artikler
Varme artikler
-
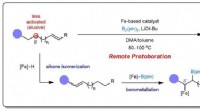 Bæredygtig jernkatalyse muliggør kontrollerbar alkenboryleringFigur 1:Skematisk viser designet af fjernprotoborering for at opnå selektiv borylering ved mindre aktiverede β-positioner. Kredit:National University of Singapore Kemikere fra National University
Bæredygtig jernkatalyse muliggør kontrollerbar alkenboryleringFigur 1:Skematisk viser designet af fjernprotoborering for at opnå selektiv borylering ved mindre aktiverede β-positioner. Kredit:National University of Singapore Kemikere fra National University -
 Forskere finder den optimale alder for stamcellerMulighedens vindue. Kredit:Daria Sokol/MIPT Biofysikere fra Moscow Institute of Physics and Technology og Vladimirsky Moscow Regional Clinical Research Institute har bestemt den optimale alder for
Forskere finder den optimale alder for stamcellerMulighedens vindue. Kredit:Daria Sokol/MIPT Biofysikere fra Moscow Institute of Physics and Technology og Vladimirsky Moscow Regional Clinical Research Institute har bestemt den optimale alder for -
 Forskere laver plastikfilm, der undertrykker coronavirusBillede af den ultrastrukturelle morfologi udstillet af 2019 Novel Coronavirus (2019-nCoV). Kredit:CDC Et konsortium bestående af firmaer Braskem, AplFilm og Nanox og universiteterne UFSCar (Brasi
Forskere laver plastikfilm, der undertrykker coronavirusBillede af den ultrastrukturelle morfologi udstillet af 2019 Novel Coronavirus (2019-nCoV). Kredit:CDC Et konsortium bestående af firmaer Braskem, AplFilm og Nanox og universiteterne UFSCar (Brasi -
 Brug af molekylær spektroskopi til at studere reaktionsmekanismerAlkylering af phenol med cyclohexanol (orange vej) sker efter, at størstedelen af cyclohexanol er dehydreret til cyclohexen. Årsagen til, at mekanismen følger denne vej, skyldes ikke begrænset adgan
Brug af molekylær spektroskopi til at studere reaktionsmekanismerAlkylering af phenol med cyclohexanol (orange vej) sker efter, at størstedelen af cyclohexanol er dehydreret til cyclohexen. Årsagen til, at mekanismen følger denne vej, skyldes ikke begrænset adgan
- Forskere viser rolle for cyanid i livets oprindelse
- Første storstilet markedsanalyse af underjordisk cyberkriminalitetsøkonomi
- Beviser fundet for regional magnetfeltanomali i Sydøstasien for 800 år siden
- Værktøjer, der bruges i Hydrology
- To døde, tusinder bedt om at flygte fra skovbranden fra Californien
- Hvad gør barometeret?


