How does the movement of air molecules cause pressure?
1. Konstant bevægelse:
- Air is composed of tiny molecules, primarily nitrogen and oxygen.
- Disse molekyler er i konstant, tilfældig bevægelse, kolliderer konstant med hinanden og med væggene i deres beholder (som indersiden af et rum eller et dæk).
2. Collisions and Force:
- Each collision between an air molecule and a surface exerts a tiny force.
- The more frequent the collisions, the greater the force.
3. Pressure:Force per Unit Area:
- Pressure is defined as the force exerted per unit area.
- Because of the constant collisions, air molecules exert a force on any surface they encounter.
- The pressure of the air is the total force of these collisions divided by the area of the surface.
4. Factors Affecting Pressure:
- Temperatur: Højere temperaturer betyder hurtigere bevægende molekyler, hvilket fører til hyppigere og kraftfulde kollisioner, hvilket resulterer i højere tryk.
- Density: More molecules in a given volume (higher density) mean more collisions, leading to higher pressure.
- Volume: Decreasing the volume of a container forces the molecules closer together, increasing the collision rate and pressure.
i enklere termer:
Imagine a swarm of bees buzzing around in a box. Each bee represents an air molecule. The bees are constantly bumping into the sides of the box. The more bees there are, and the faster they move, the more they bump into the walls, creating pressure.
Key Takeaway: Pressure in air is a result of the constant, random motion of air molecules and their collisions with surfaces.
Sidste artikelHvordan ved du, om der er sket en kemisk eller fysisk ændring?
Næste artikelHvad er anvendelserne af elektrokemiske effekter?
 Varme artikler
Varme artikler
-
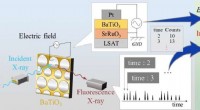 Fin struktur afsløret af potentielt alternativ til blyforbindelse, der bruges i sensorerFluorescensrøntgenstråler fra den tynde film BaTiO_3 detekteres af en solid state-detektor med tidsstempelinformation, som synkroniseres med den påførte spænding på filmen for at opnå tidsmæssig varia
Fin struktur afsløret af potentielt alternativ til blyforbindelse, der bruges i sensorerFluorescensrøntgenstråler fra den tynde film BaTiO_3 detekteres af en solid state-detektor med tidsstempelinformation, som synkroniseres med den påførte spænding på filmen for at opnå tidsmæssig varia -
 Ingeniører opdager blyfri perovskit halvleder til solceller ved hjælp af dataanalyse, supercompute…En atommodel af KBaTeBiO6 (til venstre), den mest lovende af 30, 000 oxider i et potentielt solpanel. Til højre ses et scannings transmissionselektronmikrograf, der viser atomstrukturen af KBaTeBiO6
Ingeniører opdager blyfri perovskit halvleder til solceller ved hjælp af dataanalyse, supercompute…En atommodel af KBaTeBiO6 (til venstre), den mest lovende af 30, 000 oxider i et potentielt solpanel. Til højre ses et scannings transmissionselektronmikrograf, der viser atomstrukturen af KBaTeBiO6 -
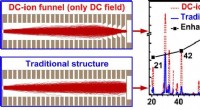 Forbedring af iontransmissionseffektiviteten af massespektrometreFokuseringseffekt af DC-ion-tragten. Kredit:Zhang Qiangling En nylig undersøgelse foretaget af forskere fra Hefei Institutes of Physical Science og offentliggjort i Analytical Chemistry præsenterer
Forbedring af iontransmissionseffektiviteten af massespektrometreFokuseringseffekt af DC-ion-tragten. Kredit:Zhang Qiangling En nylig undersøgelse foretaget af forskere fra Hefei Institutes of Physical Science og offentliggjort i Analytical Chemistry præsenterer -
 En ny måde at syntetisere antioxidante stoffer påKredit:Tomsk Polytechnic University (TPU) Forskere fra Tomsk Polytechnic University har sammen med deres kolleger fra USA og Japan foreslået en ny måde at løse den vigtigste og grundlæggende udfor
En ny måde at syntetisere antioxidante stoffer påKredit:Tomsk Polytechnic University (TPU) Forskere fra Tomsk Polytechnic University har sammen med deres kolleger fra USA og Japan foreslået en ny måde at løse den vigtigste og grundlæggende udfor
- Macau vejragentur under undersøgelse for forsinket tyfonvarsel
- To hold tester uafhængigt af hinanden Tomonaga – Luttinger -teorien
- NASA studerer delte Venus -videnskabsmål med Russian Space Research Institute
- Rapport:Amazon check-konti? Noget lignende kan snart komme
- Beregn massen af butan, der skal til for at producere 96,3 kuldioxid?
- Hvordan sammenligner vind og gletschere kontrast, når det kommer til slid på sten?


