What is the empirical formula of a compound with molecular C 12 H 8?
Here's how to find the empirical formula for C₁₂H₈:
1. Find the greatest common factor (GCD) of the subscripts: The GCD of 12 and 8 is 4.
2. Divide each subscript by the GCD:
* C₁₂ / 4 =C₃
* H₈ / 4 =H₂
Therefore, the empirical formula of the compound with molecular formula C₁₂H₈ is C₃H₂.
 Varme artikler
Varme artikler
-
 Forskere opdager nye ikke-klæbende gelerBillede af ikke-klæbende gel, der tager ved hjælp af avancerede 3D-lysmikroskopiteknikker. Kredit:University of Bristol Forskere fra University of Bristol og Université Paris-Saclay har opdaget en
Forskere opdager nye ikke-klæbende gelerBillede af ikke-klæbende gel, der tager ved hjælp af avancerede 3D-lysmikroskopiteknikker. Kredit:University of Bristol Forskere fra University of Bristol og Université Paris-Saclay har opdaget en -
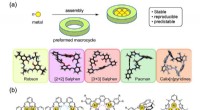 Metalliske komplekser lavet af cykliske molekylerModerne makrocykler brugt til syntese af diskrete polymetalliske komplekser:(a) koncept, (b) kemiske strukturer af repræsentative polymetalliske komplekser. Kredit:Kanazawa University I polymetall
Metalliske komplekser lavet af cykliske molekylerModerne makrocykler brugt til syntese af diskrete polymetalliske komplekser:(a) koncept, (b) kemiske strukturer af repræsentative polymetalliske komplekser. Kredit:Kanazawa University I polymetall -
 Forebyggelse af lithiumtab for højkapacitets lithium-ion-batterierKredit:Korea Institute of Science and Technology Et hold af koreanske forskere har udviklet en behandlingsteknologi til at maksimere energitætheden for batterier med høj kapacitet. Det fælles fors
Forebyggelse af lithiumtab for højkapacitets lithium-ion-batterierKredit:Korea Institute of Science and Technology Et hold af koreanske forskere har udviklet en behandlingsteknologi til at maksimere energitætheden for batterier med høj kapacitet. Det fælles fors -
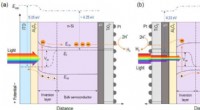 Rumlig afkobling af lysabsorption og reaktionssteder i n-Si fotokatoder til solvandssplitningSkematisk energibåndsdiagram over (a) belysningsreaktion afkoblet n-Si MIS fotokatode og (b) traditionel p-Si MIS fotokatode for HER under belysning. Kredit:Science China Press Solar-drevet fotoel
Rumlig afkobling af lysabsorption og reaktionssteder i n-Si fotokatoder til solvandssplitningSkematisk energibåndsdiagram over (a) belysningsreaktion afkoblet n-Si MIS fotokatode og (b) traditionel p-Si MIS fotokatode for HER under belysning. Kredit:Science China Press Solar-drevet fotoel
- Membranløse protoceller kunne give spor til dannelsen af tidligt liv
- Hvad er det andet navn på tredje generations sekventering?
- Hvor findes gravitationspotentiale energi?
- Hvorfor koralrev kommer i mange farver
- Hvad er forurening, der kommer fra en kilde kaldet?
- Hvad er processen, hvorpå store ark med løsnet sten bryder væk fra et outcrop?


