Den fysisk-kemiske natur af kolloide bevægelsesbølger blandt sølvkolloider
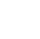
Oscillerende kolloider. (A) En polymethylmethacrylat (PMMA) mikrosfære halvcoatet med sølv (Ag) gennemgår en oscillerende kemisk reaktion mellem Ag og AgCl i nærvær af UV-lys, H2O2 og KCl (ikke vist). Indsat:scanningselektronmikrografi af PMMA-Ag Janus-sfæren; målestok, 0,5 μm. (B) En repræsentativ bane for et PMMA-Ag Janus-kolloid, der svinger mellem episoder med hurtig og langsom bevægelse. Dens øjeblikkelige hastigheder er farvekodede. Kredit:Science Advances (2022). DOI:10.1126/sciadv.abn9130
Vandrende bølger observeres almindeligvis i biologiske og syntetiske systemer, og nyere opdagelser har vist, hvordan sølvkolloider danner vandrende bevægelsesbølger i hydrogenperoxid under UV-lys. I en ny rapport, der nu er offentliggjort i Science Advances , Xi Chen og et team af forskere i smarte materialer, fysik og optik ved Harbin Institute of Technology og Shanghai Jiao Tang University i Kina, viste den kolloide bevægelsesbølge som et heterogent exciterbart system.
Sølvkolloiderne genererede vandrende kemiske bølger via reaktionsdiffusion og var enten selvkørende eller advektioneret via diffusion eller osmose. Holdet observerede de grundlæggende resultater ved hjælp af hydroxid- og pH-følsomme farvestoffer og brugte en Rogers-McCulloch-model til kvantitativt og kvalitativt at producere de karakteristiske træk ved kolloide bølger. Resultaterne baner vejen for at integrere kolloide bølger som en platform til at studere ikke-lineære fænomener og undersøge kolloid transport for at udforske informationstransmission i biomimetiske mikrorobottensembler.
Oversættelse af biologisk oscillation i laboratoriet
Oscillatoriske processer er almindeligt observeret i levende systemer, varierende fra døgnrytmen til cytosoliske oscillationer. Koblingen mellem oscillerende enheder kan føre til synkronisering, der giver anledning til vandrende bølger, som observeret med calciumbølger, der spredes over et befrugtet æg, aktionspotentialer, der forplanter sig over bankende hjerteceller, mitotiske tilstande og bølger af selvorganiserende amøber. Biofysikere sigter mod at forstå den fysisk-kemiske natur af disse bølger for at undersøge de underliggende tendenser i livet. Nylige opdagelser af de fotokemisk aktive, sølvholdige oscillerende kolloider er en spændende tilføjelse til familien af ikke-lineære processer.
Da forskere nedsænkede en inert polymermikrosfære halvbelagt med sølv i en vandig opløsning af hydrogenperoxid eller kaliumchlorid og udsatte dem for lyskilder, bemærkede de visningen af impulser. De foreslog, at de sølvnanopartikler, der blev produceret under eksperimentet, tjente som katalytiske hotspots for at muliggøre yderligere reaktioner. Uanset den kemiske detalje bemærkede holdet, hvordan diffusion af kemikalier drev Janus-partiklerne frem via selvdiffusioforese for at give anledning til lignende kolloide bevægelser. I dette arbejde tilbød Chen et al. et første syn på at generere de kemisk aktive kolloider og overvågede deres respons på kemiske bølger ud over de klassiske reaktionsdiffusionssystemer. Resultaterne giver stærke muligheder for translationel forskning, der forbinder aktivt stof med ikke-lineær videnskab for at regulere sværme af biomimetiske mikroskopiske maskiner.
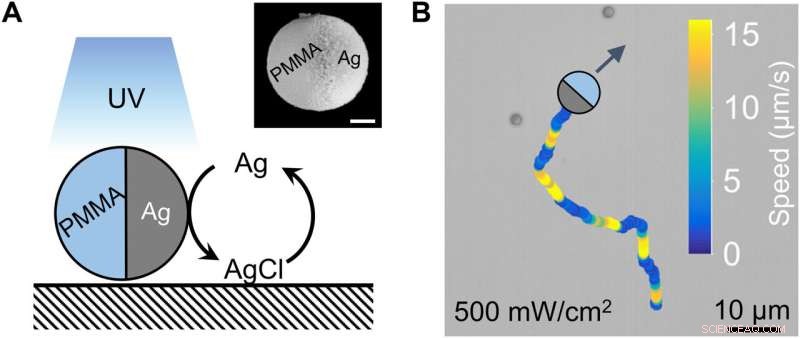
Holdet bemærkede udviklingen af periodiske kolloide bevægelsesbølger i synkroniseret udbredelse. De havde tidligere registreret ballistiske bølger ved en mellemliggende befolkningstæthed, hvor aktiverede kolloider ved bølgefronten bevægede sig i alle retninger på grund af foretisk selvfremdrift. Forskerne bemærkede fremkomsten af kvalitativt forskellige typer bølger, kendt som sværmende bølger ved endnu højere befolkningstætheder. I dette tilfælde udviklede holdet polymethylmethacrylat-mikrosfærer halvcoatet med sølv (PMMA-Ag), suspenderet i hydrogenperoxid og kaliumchlorid og belyst med 365 nm lys. Den kolloide partikel indeholdende sølv kunne i princippet udsende sværmende bølger. De eksperimentelle resultater indikerede et resultat svarende til den "mexicanske bølge" set på fodboldstadioner. Holdet kvantificerede derefter den sværmende bølge via sporing af enkeltpartikler og mikropartikel-billedhastighed ved at betragte de kolloide partikler som strømningssporere. I dette tilfælde rejste bølgen med en hastighed på 16 µm/s med indstillelige parametre. Ændringer i lysintensitet ændrede kun mildt perioden og hastighederne for en sværmende bølge. Holdet skelnede de sværmende bølger fra ballistiske bølger via deres karakteristiske mobilitet og fysikokemi
Ballistic and swarming colloidal waves. (A) Schematic diagram of a colloidal motion wave propagating to the right. Each sphere is half-coated with Ag that is not drawn. (B) Ballistic wave propagating across a population of PMMA-Ag colloids. Activated colloids are marked with red dots and their velocities are labeled with arrows. ϕ =1.3%. This figure came from figure 1D in (27). Copyright 2021, Royal Society of Chemistry. (C) Swarming wave propagating downward. Particle velocities are labeled with arrows, so that those moving toward an incoming wave are in orange and those trailing a wave are in dark blue. ϕ =29%. Kredit:Science Advances (2022). DOI:10.1126/sciadv.abn9130
Chemical waves:The physicochemical nature of a colloidal wave
Chen et al described the physicochemical nature of the activation and recovery of colloidal waves. Since the wave phenomenon is inspired by traveling waves in reaction-diffusion systems, they hypothesized colloidal waves to be underpinned by a traveling chemical wave, due to reaction-diffusion mechanisms. For instance, hydrogen peroxide can decompose faster in higher pH to form a burst of highly oxidative intermediates that oxidized silver into silver chloride. The resulting chemical reactivity activated the silver-colloid to release a burst of chemicals to maintain chemical wave propagation. They confirmed the production of hydroxide anions during silver oxidation, and the formation of hydrogen cations during silver chloride photodecomposition, at and behind the chemical wavefront by using fluorescence mapping and pH measurements.
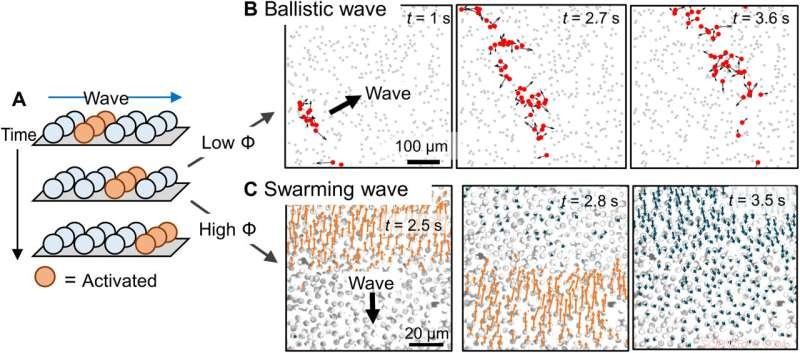
Quantitative characterization of a swarming wave. (A) Micro-PIV–generated flow velocities along the y direction (Vy) of a population of PMMA-Ag particles during the downward propagation of a wave. Positive (upward) velocities are colored red and negative velocities are colored blue. Cartoons in the insets represent how colloids move at or after a wavefront. (B) Normalized Vy averaged across the rectangular box labeled in (A) during the downward propagation of three consecutive waves. Wave periods are calculated by finding the time differences between the peaks. (C) Normalized flow velocities averaged over x at four different time instances labeled in (A) as one wave propagates along y. Wave speed, Vwave, is calculated by dividing the distance the wavefront travels along y (∆ypeak) by the time interval ∆t. (D) Wave periods and speeds under different light intensities. (E) Wave speeds at different population densities ϕ. (F) Particle speeds at different population densities. Error bars represent SDs from three measurements; 0.5 wt % H2O2 and 200 μM KCl were used in all experiments in this figure. Kredit:Science Advances (2022). DOI:10.1126/sciadv.abn9130
Colloids respond to a chemical wave:Modeling a reaction-diffusion colloidal wave
The scientists next studied the dynamics of colloidal particles in a chemical wave to dictate the type of colloidal wave formed. They noted ionic self-diffusiophoresis, and at higher ionic densities they noted weaker electro-kinetic effects for reduced self-propulsion. They identified the dynamics of neutral diffusio-osmosis dynamics, which moved colloid particles via advection, in addition to self-propagation. As self-propagation weakened and diffusio-osmosis intensified in a crowded solution with rising ionic strength, the colloidal wave switched to swarming wave. The team observed a range of effects, including electrokinetic effects, advection support via osmosis, and self-propulsion during the experiments. Chen et al next reproduced and corroborated the proposed reaction-diffusion colloidal wave via numerical simulations. At the first step, they used the Rogers-McCulloch model to simulate a chemical wave, the resulting numerical models qualitatively reproduced key features, to explore the dynamics of colloidal waves.
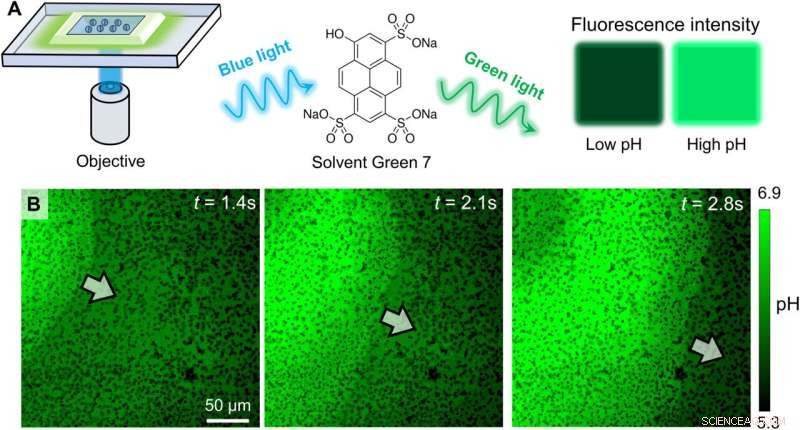
Experimental confirmation of an OH− wave. (A) Schematic diagram of the experimental setup for relating the fluorescence emission of Solvent Green 7 with local pH. (B) Optical micrographs of the pH profile during the propagation of a colloidal wave. PMMA-Ag particles of a population density ϕ of 25% were suspended in an aqueous solution containing 0.5 wt % H2O2, 200 μM KCl, and 100 μM Solvent Green 7. A blue light source (475 nm, 75 mW/cm2) served both to activate the oscillatory reaction and to excite the dye molecules. Kredit:Science Advances (2022). DOI:10.1126/sciadv.abn9130
Qualitative comparison of colloidal waves between experiments (left) and simulations (right). (A to D) Evolution of target waves (A and B) and spiral waves (C and D). (E and F) The annihilation of two colloidal waves traveling in opposite directions. (G and H) Two consecutive waves. In all experiments, PMMA-Ag particles [population density ϕ of 20% for (B), 15% for (D), 20% for (F), and 23% for (H)] were suspended in an aqueous solution containing 0.5 wt % H2O2 and 200 μM KCl under a 405-nm illumination of 1.6 W/cm2. Kredit:Science Advances (2022). DOI:10.1126/sciadv.abn9130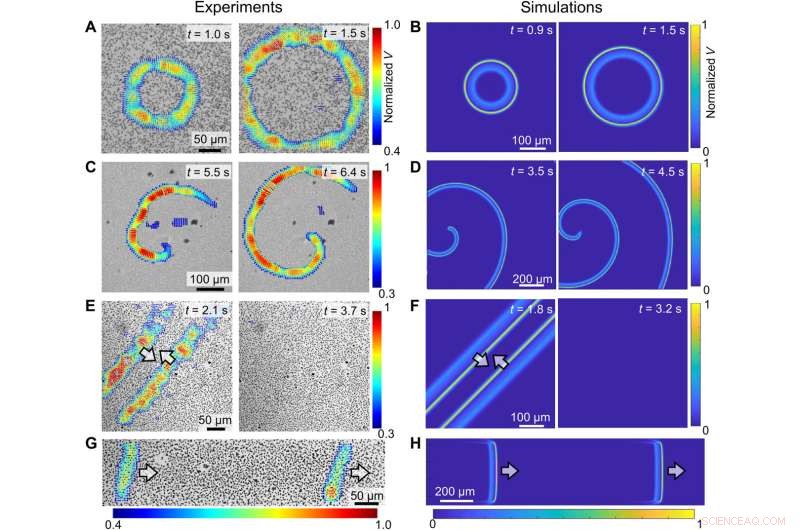
In this way, Xi Chen and colleagues developed a numerical model to simulate colloidal waves to study the heterogeneity of chemical waves. The outcomes showed good agreement with simulations and experiments to provide key insights to understand microscopic details of chemical waves in experimental systems. Colloidal waves can be integrated with optical tweezers, acoustofluidics or microfluidics to regulate micro- and nanoscopic objects in space and time. The method is useful to swarm physicochemical dynamics of a colloidal wave and can lead to develop wave-mediated information transmission systems to examine autonomous micro-robots. The colloidal waves present a good model system of reaction-diffusion processes at mesoscopic and microscopic scales. + Udforsk yderligere
Axisymmetric 'spike waves' far exceed limits previously thought to dictate maximum height of ocean waves
© 2022 Science X Network
 Varme artikler
Varme artikler
-
 Forskere gør fascinerende fjerfundPapegøjefjerlaser:En laser sonderer en papegøjefjer, afslører dets pigmentkomponenter. Kredit:Jonathan Barnsley Har du nogensinde spekuleret på, hvor papegøjer får deres lyse fjerdragt? Et Otago-l
Forskere gør fascinerende fjerfundPapegøjefjerlaser:En laser sonderer en papegøjefjer, afslører dets pigmentkomponenter. Kredit:Jonathan Barnsley Har du nogensinde spekuleret på, hvor papegøjer får deres lyse fjerdragt? Et Otago-l -
 Forskere finder en måde at kontrollere, at kvantecomputere returnerer nøjagtige svarFlere kvantecomputere, der bruger forskellig hardware, testes mod hinanden ved at lade dem udføre tilfældige beregninger, som er forbundet med en skjult grafstruktur. Kredit:Ella Maru Studio Kvant
Forskere finder en måde at kontrollere, at kvantecomputere returnerer nøjagtige svarFlere kvantecomputere, der bruger forskellig hardware, testes mod hinanden ved at lade dem udføre tilfældige beregninger, som er forbundet med en skjult grafstruktur. Kredit:Ella Maru Studio Kvant -
 Hvordan urans elektroniske egenskaber og atomvibrationer er forbundetEn elektronisk ustabilitet destabiliserer gitteret, udløser ladningstæthedsbølge og inducerer Kohn-anomali. Kredit:Aditya Prasad Roy, Institut for Mekanik, IIT Bombay Forskere har forklaret, hvord
Hvordan urans elektroniske egenskaber og atomvibrationer er forbundetEn elektronisk ustabilitet destabiliserer gitteret, udløser ladningstæthedsbølge og inducerer Kohn-anomali. Kredit:Aditya Prasad Roy, Institut for Mekanik, IIT Bombay Forskere har forklaret, hvord -
 Kemikere beskriver en ny form for isKredit:Pavel Odinev / Skoltech Forskere fra USA, Kina og Rusland har beskrevet strukturen og egenskaberne af et nyt hydrogenclathrathydrat, der dannes ved stuetemperatur og relativt lavt tryk. Hyd
Kemikere beskriver en ny form for isKredit:Pavel Odinev / Skoltech Forskere fra USA, Kina og Rusland har beskrevet strukturen og egenskaberne af et nyt hydrogenclathrathydrat, der dannes ved stuetemperatur og relativt lavt tryk. Hyd
- Lidt af et stræk... materiale, der bliver tykkere, når det trækkes
- Ny tiltrækkende millisekund røntgenpulsar opdaget
- Astronomer opdager store gamle galakser, som kunne kaste lys over mørkt stof
- Ripple effect:Undersøgelse afslører NYS æbleindustriens sande økonomiske indvirkning
- Sådan undervises børn i det grundlæggende i procent
- Juryen fortæller, at Samsung skal betale stort for at kopiere iPhone-design