Elektrofotokatalytisk diamination af vicinale C -H -bindinger
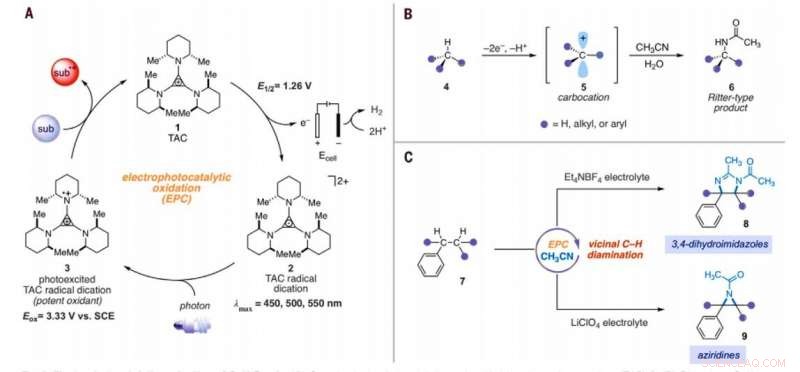
Elektrofotokatalytisk amination af C -H -bindinger. (A) Generisk elektrofotokatalytisk cyklus med trisaminocyclopropenium (TAC) 1. (B) Ritter-type C-H amineringsreaktion. (C) Elektrofotokatalytiske vicinale C -H -diamineringsreaktioner rapporteret i dette arbejde. Sub, substrat; subox, oxideret substrat; Mig, methyl; Et, ethyl; Ac, acetyl; Eox, oxidationspotentiale; lmax, bølgelængde med maksimal absorption. Kredit:Videnskab, 10.1126/science.abf2798
I organisk kemi, omdannelsen af inaktiverede carbon-hydrogen (C-H) bindinger til carbon-nitrogen (C-N) bindinger er en højt værdsat transformation. Forskere kan udføre sådanne reaktioner på kun et enkelt CH-sted, da den første derivatisering kan reducere reaktiviteten af de omgivende CH-bindinger. I en ny rapport nu offentliggjort i Videnskab , Tao Shen og Tristan H. Lambert ved afdelingen for kemi og kemisk biologi, Cornell University, New York, viste, at alkylerede arenaer kunne undergå vicinale CH-diamineringsreaktioner til form 1, 2-diaminderivater ved anvendelse af en elektrofotokatalytisk strategi. Under den syntetiske proces, de brugte acetonitril som opløsningsmiddel og nitrogenkilde. De katalyserede reaktionen ved hjælp af en trisaminocyclopropenium (TAC) ion, som gennemgik anodisk oxidation for at give en stabil radikal dikation (enhver kation), mens den katodiske reaktion reducerede protoner til molekylært brint. Da de bestrålede TAC-radikaldikationen med et hvidt lys, kompakt fluorescerende lys, de genererede et stærkt oxiderende fotoexciteret mellemprodukt. Baseret på den anvendte elektrolyt, holdet opnåede enten 3, 4-dihydroimidazol eller aziridinprodukter.
En ny syntetisk proces
Allestedsnærværende kemiske reaktioner, der konverterer inerte carbon-hydrogen (C-H) bindinger til værdifulde carbon-nitrogen (C-N) bindinger, kan i høj grad fremskynde konstruktionen af komplekse molekyler, der er relevante for den biomedicinske virksomhed. Forskere har derfor udledt en række CH-amineringsreaktioner, men på trods af deres magt og omfang, mange syntetiske kampagner skal installere mange C-N-forbindelser. En stor udfordring for at udvikle kemiske sådanne reaktioner er, at heterofunktionalitet har en tendens til at deaktivere omgivende bindinger mod de typiske mekanistiske former for C -H -aktivering. Kun få reaktionsteknologier har hidtil derfor udført multipotente funktioner på proksimale CH-bindinger. Shen et al. beskrev en strategi for potent oxidationskemi ved at kombinere lysets og elektricitetens energi i en enkelt katalysator i en proces kendt som elektrofotokatalyse (EPC). Under denne strategi, holdet brugte elektrokemisk oxidation af trisaminocyclopropenium (TAC) ion under et relativt mildt elektrokemisk potentiale og samtidig synlig lysbestråling for at ophidse det resulterende radikale kationiske mellemprodukt. Den foto-ophidsede radikale dikering var en ekstremt kraftig oxidant, der demonstrerede udfordrende reaktioner, herunder oxidative funktioner af benzen og andre elektronfattige arenaer eller regioselektiv C-H-funktionalisering af etere.
Substratomfang af elektrofotokatalytisk vicinal C -H -diamination. Alle udbytter er isolerede produkter. Produkter blev opnået som racemiske blandinger; kile- og stregafbildninger angiver relative stereokemiske forhold. (A) Diamination af sekundære alkylbenzener. (B) Diamination af primære alkylbenzener. Eksperimentelle detaljer findes i de supplerende materialer. En stjerne angiver kørsel ved 2,2 V; et dolkesymbol (†) angiver oparbejdning med NaHCO3 (aq) og CH3OH; og en dobbelt dolk dymbol (‡) angiver nBu4NPF6 i stedet for Et4NBF4. SM, udgangsmaterialer. Forbindelse 36 blev deacyleret ved oparbejdning. Kredit:Videnskab, 10.1126/science.abf2798 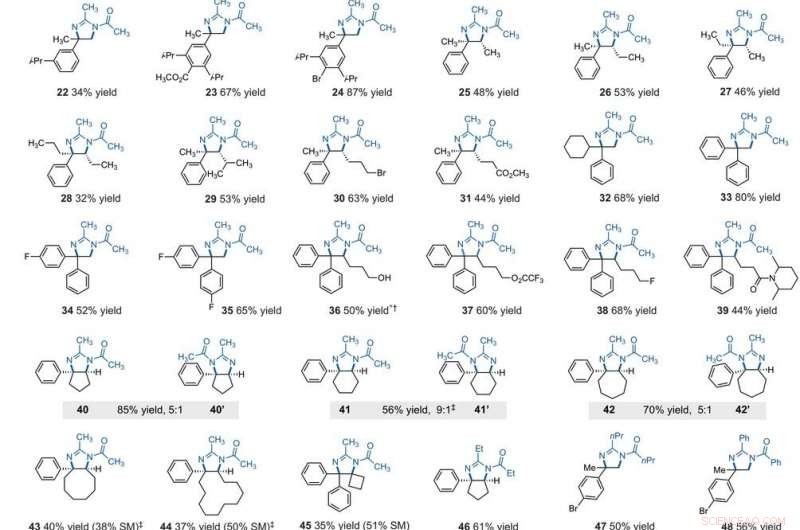
Teamet antog TACs oxiderende kraft til også at muliggøre andre CH-bindingsaktiveringsmanifold. Under de rigtige betingelser, den elektrofotokatalytiske tilgang kunne generere carbo-kation-mellemprodukter for at lette Ritter-funktionalisering af C-H-bindinger uden en ekstern kemisk oxidant. Typisk, under Ritter-type reaktioner genererer en carbokation med efterfølgende indfangning af et nitril for at danne nitriliumion-mellemprodukter efterfulgt af amidprodukter efter hydrolyse. Teamet antog, at de stærkt oxiderende, alligevel kan selektive betingelser tilbydes af TAC (trisaminocyclopropenium) EPC (elektrofotokatalyse) muliggøre en sekvens af flere Ritter-type H-funktionaliseringsreaktioner, hvor den oprindeligt dannede acetamidgruppe lettede en anden amineringsreaktion i en tilstødende position. Hvis det er muligt, metoden kunne lette regioselektiv amination af to C-H-bindinger blot ved hjælp af synligt lys, et mildt elektrokemisk potentiale og et almindeligt opløsningsmiddel som nitrogenkilden i stedet for nitrenforstadier. Shen et al. reported the realization of the electrophotocatalytic deamination of C-H bonds to furnish dihydroimidazoles or aziridines, depending on the type of electrolyte used during the experiments.
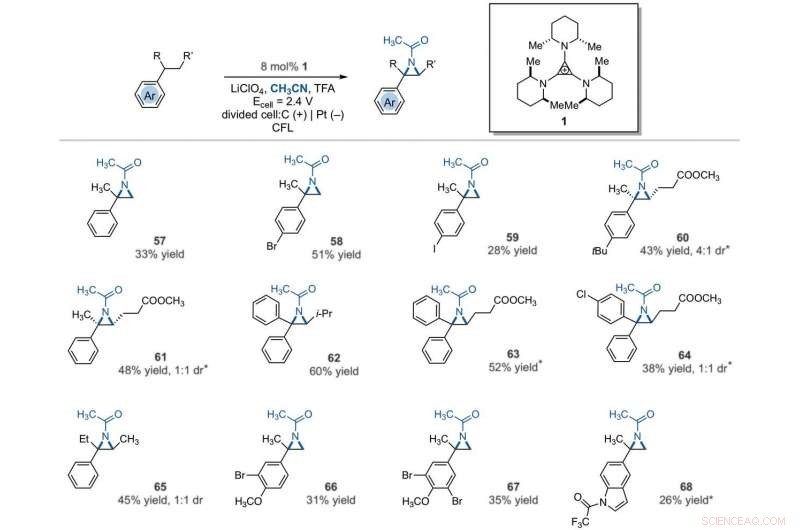
Electrophotocatalytic vicinal C–H aziridination. Detailed reaction conditions for each substrate are provided in the supplementary materials. Products were obtained as racemic mixtures; wedge and dash depictions indicate relative stereochemical relationships. An asterisk indicates run at 2.2 V. i-Pr, isopropyl. Kredit:Videnskab, 10.1126/science.abf2798
The synthetic products
After extensively screening, the reaction conditions including the cell potential, electrolyte, acid additive and reaction time, Shen et al. identified conditions to assist the efficient conversion of a variety of benzylic hydrocarbons of the corresponding N-acyl-4, 5-dihydroimidazole adducts. In the reaction setup, the scientists used visible light irradiation with a white compact fluorescent light of a solution of the substrate containing TAC in a divided electrolytic cell under controlled potential. The team added the TAC catalyst and substrate within the anodic chamber where the C-H deamination chemistry occurred. The resulting redox by-product was effectively traceless. Based on similar conditions, a variety of benzylic hydrocarbons underwent vicinal C-H diamination to form diverse products. I alle tilfælde, the researchers noted how the function of methylene carbons occurred in preference to methyl carbons, even when in the presence of a sterically demanding group or electron-withdrawing group. Since the α-α-diaryl amines formed a valuable substructure in biomedically relevant compounds, the team also investigated the transformation on gem-diaryl substrates. They found that the 1, 1-diphenyl ethane reacted efficiently to furnish a secondary alkyl benzene compound with 80 percent yield. The compatibility of alcohol, ester, alkyl fluoride and amide substituents allowed the synthesis of more highly functionalized adducts.
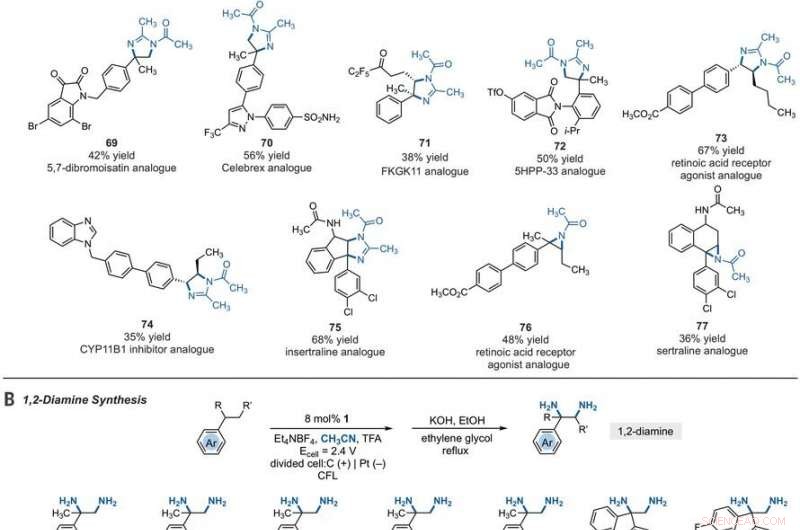
Synthetic applications of electrophotocatalytic vicinal C–H diamination. (A) Bioactive compound analogs prepared by means of electrophotocatalytic vicinal C–H diamination or aziridination. (B) 1, 2-Diamine synthesis. (C) Dihydroimidazole synthesis. (D) Bioactive compound synthesis. Detailed reaction conditions are provided in the supplementary materials. Products were obtained as racemic mixtures; wedge and dash depictions indicate relative stereochemical relationships. Products 80 and 81 were isolated as bis tosylate salts. Ph, phenyl; Tf, trifluoromethanesulfonate. Kredit:Videnskab, 10.1126/science.abf2798
Functionalizing ring systems
The team further studied the potential of this reaction to functionalize ring systems. The reaction of phenyl cyclopentane led to the bicyclic compound in 85 percent yield. The scientists produced six- and seven-membered ring products as regioisomeric mixtures, alongside eight- and 12-membered ring products as single isomers. They improved some of the yields for cyclic substrates by using tetrabutylammonium phosphate (TBAF 6 ) as the electrolyte. In addition to acetonitrile, the researchers used other nitriles to give rise to diaminated products derived from propionitrile, butyl nitrile or benzonitrile as the nitrogen source. The scientists also tested the diamination process using unbranched benzylic substrates. Som resultat, imine and halogenated derivatives gave rise to aziridines in low to modest yields with nearly equal yields of diaminated products.
Mechanistic rationale for electrophotocatalytic vicinal C–H diamination. Voltages were measured in a 5:1 mixture of CH3CN and TFA to mimic the reaction conditions and are relative to SCE. Kredit:Videnskab, 10.1126/science.abf2798 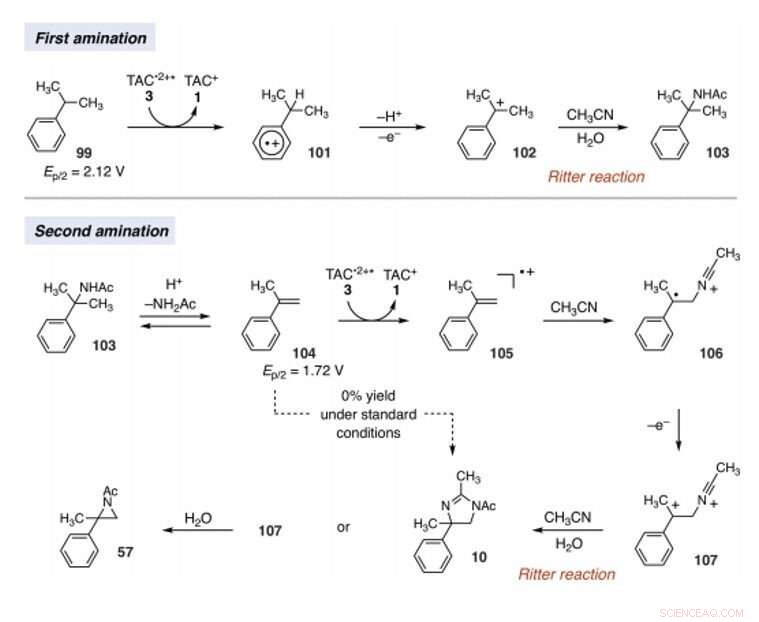
Since late-stage C-H functionalization processes offered powerful tools for the diversification of medicinal compound libraries, Shen et al. tested the difunctionalization chemistry on several molecules that are close analogs of known biologically active molecules. The team diaminated a dibromoisatin derivative to produce a bioactive molecule analogue in 42 percent yield. For eksempel, Isatin derivatives have been investigated in the past due to their medicinal properties including antitumor and antiviral activities. The scientists also found that celecoxib analogs could produce 56 percent yield under standard conditions. They then converted an analog of thalidomide with antiproliferative activity into another bioactive analog with 50 percent yield. The team further found how a small modification to the electrophotocatalytic procedure could lead to the isolation of free 1, 2-diamines in good yields. Shen et al. believe the mechanisms to have originated with Ritter-type amination of the substrates benzylic C-H bond in a process that accords with known electrochemical Ritter-type reactions. På denne måde, Tao Shen and Tristan H. Lambert noted the compatibility of deamination with a reasonable diversity of functionality for the practical applications of this reaction. The scientists used the power of combined light and electrical energy to conduct the reactions in the functionality of a single catalyst with advancing synthetic capabilities.
© 2021 Science X Network
 Varme artikler
Varme artikler
-
 En vandspaltende katalysator ulig nogen andenKredit:CC0 Public Domain Elektricitet kan genereres af vedvarende kilder som sollys og vind, derefter brugt til at spalte vand, som gør brint som brændstof til nye energienheder såsom brændselscel
En vandspaltende katalysator ulig nogen andenKredit:CC0 Public Domain Elektricitet kan genereres af vedvarende kilder som sollys og vind, derefter brugt til at spalte vand, som gør brint som brændstof til nye energienheder såsom brændselscel -
 Brintproduktion i et begrænset rumFigur 1:Indkapslingen af ædelmetal nanopartikler i MoS2 ved en in-situ reduktion strategi. National University of Singapore kemikere har udviklet en metode til at begrænse ædelmetal -nanopartikl
Brintproduktion i et begrænset rumFigur 1:Indkapslingen af ædelmetal nanopartikler i MoS2 ved en in-situ reduktion strategi. National University of Singapore kemikere har udviklet en metode til at begrænse ædelmetal -nanopartikl -
 Protocellgæster flygter fra redenKonfokal fluorescensmikroskopibillede, der viser et tværsnit af en vært-gæst nestet protocelle bestående af et fanget gæstproteinosom (grønt), der er fanget inde i en vært-coacervat mikrodråbe (rød).
Protocellgæster flygter fra redenKonfokal fluorescensmikroskopibillede, der viser et tværsnit af en vært-gæst nestet protocelle bestående af et fanget gæstproteinosom (grønt), der er fanget inde i en vært-coacervat mikrodråbe (rød). -
 Ultraviolet lys-baseret belægning viser løfte i selvdesinficerende overflader i medicinske facilit…Kredit:CC0 Public Domain Verdenssundhedsorganisationen advarer om, at antibiotikaresistens er en af de største globale trusler og forudsiger, at verdensomspændende dødsrater fra denne trussel ka
Ultraviolet lys-baseret belægning viser løfte i selvdesinficerende overflader i medicinske facilit…Kredit:CC0 Public Domain Verdenssundhedsorganisationen advarer om, at antibiotikaresistens er en af de største globale trusler og forudsiger, at verdensomspændende dødsrater fra denne trussel ka
- Polymer videnskabsmænd jammer nanopartikler, at fange væsker i nyttige former
- Sådan beregnes området for en lige-sidet trekant
- Hvad forårsager dag /natcyklus på jorden?
- Kold, tørre planeter kan have mange orkaner
- En ny metode til at opdage falske datainjektion (FDI) angreb
- Ansigtsgenkendelsesforbud:Hvad er det næste i Oakland, hos Amazon og mere


