Hvad er den procentvise sammensætning af CH3CH2OH?
1. Bestem den molære masse for hvert element:
* Carbon (c):12,01 g/mol
* Hydrogen (H):1,01 g/mol
* Oxygen (O):16,00 g/mol
2. Beregn den molære masse af hele molekylet:
* CH3CH2OH:(2 x 12,01) + (6 x 1,01) + 16,00 =46,07 g/mol
3. Beregn masseprocenten for hvert element:
* carbon (c): [(2 x 12,01) / 46,07] x 100% = 52,14%
* brint (H): [(6 x 1,01) / 46,07] x 100% = 13,13%
* ilt (O): [16.00 / 46.07] x 100% = 34,73%
Derfor er den procentvise sammensætning af CH3CH2OH:
* kulstof:52,14%
* brint:13,13%
* ilt:34,73%
 Varme artikler
Varme artikler
-
 Selvkørende, endeløst programmerbare kunstige ciliaKredit:Harvard University I årevis har forskere forsøgt at konstruere små, kunstige cilia til miniature robotsystemer, der kan udføre komplekse bevægelser, herunder bøjning, vridning og vending. At
Selvkørende, endeløst programmerbare kunstige ciliaKredit:Harvard University I årevis har forskere forsøgt at konstruere små, kunstige cilia til miniature robotsystemer, der kan udføre komplekse bevægelser, herunder bøjning, vridning og vending. At -
 Kemisk binding versus elektromagnetiske bølgerKredit:Leiden Universitet Vibrerende kuliltemolekyler adsorberet ved overfladen af en saltkrystal holder op med at bevæge sig efter et par millisekunder. Forskere har nu opdaget, at dette er dom
Kemisk binding versus elektromagnetiske bølgerKredit:Leiden Universitet Vibrerende kuliltemolekyler adsorberet ved overfladen af en saltkrystal holder op med at bevæge sig efter et par millisekunder. Forskere har nu opdaget, at dette er dom -
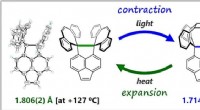 Kulstof-kulstof kovalente bindinger langt mere fleksible end antagetMolekylerne syntetiseret og analyseret af forskergruppen. I grøn og blå er den fleksible C-C enkeltbinding (Takuya Shimajiri, Takanori Suzuki, Yusuke Ishigaki, Angewandte Chemie International Edition,
Kulstof-kulstof kovalente bindinger langt mere fleksible end antagetMolekylerne syntetiseret og analyseret af forskergruppen. I grøn og blå er den fleksible C-C enkeltbinding (Takuya Shimajiri, Takanori Suzuki, Yusuke Ishigaki, Angewandte Chemie International Edition, -
 Video:Den mærkelige kemi, der truer mesterværksmalerierKredit:The American Chemical Society En god kunsthandler kan virkelig rydde op på dagens marked, men ikke når en eller anden underlig kemi ødelægger mesterværker. Kunstkonservatorer begyndte at l
Video:Den mærkelige kemi, der truer mesterværksmalerierKredit:The American Chemical Society En god kunsthandler kan virkelig rydde op på dagens marked, men ikke når en eller anden underlig kemi ødelægger mesterværker. Kunstkonservatorer begyndte at l
- Hvad beskriver alle eksisterende bakterier?
- At kaste lys over naturens kontrol af solenergi på nanoskala
- Truende tyfon Yutu undersøgt af GPM Satellite
- Hvad er de fire principper klasser af organiske forbindelser?
- Hvad er elektronerne i den indre skal?
- WAT SALT er ansvarlig for midlertidig og permanent hårdhed af vand?


