Hvad er den molære masse af Mercurous Nitrate Hg2NO3 2?
* hg (Mercury): 200,59 g/mol * 2 =401,18 g/mol
* n (nitrogen): 14,01 g/mol * 2 =28,02 g/mol
* o (ilt): 16,00 g/mol * 6 =96,00 g/mol
Total molmasse =401,18 g/mol + 28,02 g/mol + 96,00 g/mol =525,20 g/mol
Derfor er den molære masse af Mercurous nitrat, HG₂ (NO₃) ₂, 525,20 g/mol .
 Varme artikler
Varme artikler
-
 Smarte magnetiske bløde materialer til udvikling af kunstige muskler og terapeutiske robotterInteraktionskræfter mellem magnetiske partikler omsættes til makroskopiske transformationer af de smarte polymerer. Kredit:4D-BIOMAP Udvikling af en ny generation af kunstige muskler og bløde nano
Smarte magnetiske bløde materialer til udvikling af kunstige muskler og terapeutiske robotterInteraktionskræfter mellem magnetiske partikler omsættes til makroskopiske transformationer af de smarte polymerer. Kredit:4D-BIOMAP Udvikling af en ny generation af kunstige muskler og bløde nano -
 Porøse polymerbelægninger styrer lys og varme dynamiskDe porøse polymerbelægninger, der skifter fra hvidt til gennemsigtigt, når det er fugtet, kan sættes i plastikskabe for at lave paneler, der styrer lys og temperaturer i bygninger. Kredit:Jyotirmoy Ma
Porøse polymerbelægninger styrer lys og varme dynamiskDe porøse polymerbelægninger, der skifter fra hvidt til gennemsigtigt, når det er fugtet, kan sættes i plastikskabe for at lave paneler, der styrer lys og temperaturer i bygninger. Kredit:Jyotirmoy Ma -
 Ny teknik til at skabe super-svampe er en game changerForskere har udtænkt nye metoder til postsyntetisk modifikation af metal-organiske rammer for at producere egenskaber i materialet, der er ideelt til gasmanipulation. Kredit:DGIST Metal-organiske
Ny teknik til at skabe super-svampe er en game changerForskere har udtænkt nye metoder til postsyntetisk modifikation af metal-organiske rammer for at producere egenskaber i materialet, der er ideelt til gasmanipulation. Kredit:DGIST Metal-organiske -
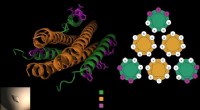 Forskere udvikler en bedre måde at blokere vira, der forårsager luftvejsinfektioner i barndommenDet antivirale peptid består af tre proptrækkere (i grønt), som låser sig omkring virussens fusionsprotein (i orange) for at forhindre virussen i at trænge ind i cellerne. Gellman-laboratoriet tilføje
Forskere udvikler en bedre måde at blokere vira, der forårsager luftvejsinfektioner i barndommenDet antivirale peptid består af tre proptrækkere (i grønt), som låser sig omkring virussens fusionsprotein (i orange) for at forhindre virussen i at trænge ind i cellerne. Gellman-laboratoriet tilføje
- Sådan tuner du almindelige lugte og fokuserer på vigtige
- Hvem studerer variationen i hastighed?
- Hvor i naturen kunne du finde en kilde til den samme energi som den, der leveres af batterier?
- Når vandige opløsninger af natriumfluorid og hydrobrominsyre blandes en opløsningsbromidhydrofluo…
- I en kemisk reaktion er den samlede masse af reaktanter 10,0 gram produkterne skal være?
- Hvad betyder Mg /D?


