Hvor mange gram brint er der i 46G CH4O?
1. Bestem den molære masse af CH4O:
* Carbon (c):12,01 g/mol
* Hydrogen (H):1,01 g/mol
* Oxygen (O):16,00 g/mol
Molmasse af CH4O =12,01 + (4 * 1,01) + 16,00 =32,05 g/mol
2. Beregn masseprocenten af brint i CH4O:
* Mass af brint i CH4O:4 * 1,01 g/mol =4,04 g/mol
* Masseprocent af brint =(4,04 g/mol/32,05 g/mol) * 100% =12,61%
3. Beregn gram af brint i 46 g Ch4O:
* Gram brint =(12,61% / 100%) * 46g = 5,81g
Derfor er der cirka 5,81 gram brint i 46 gram ch4o.
 Varme artikler
Varme artikler
-
 For at lave aminosyrer, tilføj bare elektricitetEn demonstrationsflowreaktor konstrueret af forskere ved Kyushu University omdanner løbende kildematerialer til aminosyrer gennem en reaktion drevet af elektricitet. Ved at vælge den rigtige kombinati
For at lave aminosyrer, tilføj bare elektricitetEn demonstrationsflowreaktor konstrueret af forskere ved Kyushu University omdanner løbende kildematerialer til aminosyrer gennem en reaktion drevet af elektricitet. Ved at vælge den rigtige kombinati -
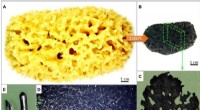 Ekstrem biomimetik - søgen efter naturlige kilder til materialeteknisk inspirationOversigt over transformationen af svampestilladser til en karboniseret 3D -struktur ved 1200 ° C. (A) Typisk cellulær og hierarkisk morfologi af Hippospongia communis demosponge organisk skelet efte
Ekstrem biomimetik - søgen efter naturlige kilder til materialeteknisk inspirationOversigt over transformationen af svampestilladser til en karboniseret 3D -struktur ved 1200 ° C. (A) Typisk cellulær og hierarkisk morfologi af Hippospongia communis demosponge organisk skelet efte -
 Kemisk billeddannelse i stort område afslører originale malingslag på altertavlen i GentKredit:Wiley Betragtes som toppen af middelalderlig maleri, Gent -altertavlen blev malet omkring 1432 af Jan van Eyck og sandsynligvis hans bror Hubert. Det gennemgår i øjeblikket den mest omfat
Kemisk billeddannelse i stort område afslører originale malingslag på altertavlen i GentKredit:Wiley Betragtes som toppen af middelalderlig maleri, Gent -altertavlen blev malet omkring 1432 af Jan van Eyck og sandsynligvis hans bror Hubert. Det gennemgår i øjeblikket den mest omfat -
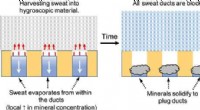 Vende sveden mod sig selv med en metalfri antiperspirantKredit:American Chemical Society Kropslugt er en ubehagelig lugt, produceres, når bakterier, der lever på huden, nedbryder proteinerne i sved. For at undgå at stinke, nogle mennesker anvender anti
Vende sveden mod sig selv med en metalfri antiperspirantKredit:American Chemical Society Kropslugt er en ubehagelig lugt, produceres, når bakterier, der lever på huden, nedbryder proteinerne i sved. For at undgå at stinke, nogle mennesker anvender anti
- Eddike og bagepulver:Et rengøringshack eller bare en masse sodavand?
- Hvilken planet har det korteste år med indre planeter?
- Hvorfor vokser formen på frugt og brød?
- NASAs Parker Solar Probe kaster nyt lys på solen
- Er kovalent det samme som makromolekylært?
- Hvordan er temparatur relateret til kenetisk energi af partikler?


