Vand, vand overalt:Polarisering påvirker dramatisk H2O -strukturen afsløret gennem molekylær dynamiksimulering
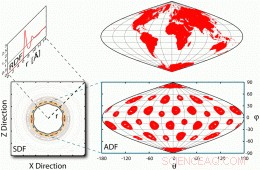
Forskellige fordelingsfunktioner for et vandmolekyle. Tre forskellige måder at måle rækkefølgen af vand O- og H -atomer omkring C60 eller faktisk et hvilket som helst opløst stof. Mest indlysende er den tredimensionelle rumlige fordelingsfunktion (SDF), der viser tætheden ved hvert volumenelement centreret på hvert punkt i rummet defineret af koordinater (x, y, z). Det lider af de alvorlige vanskeligheder forbundet med visning og analyse af et generelt tredimensionelt kort. Mest almindeligt anvendt er den endimensionelle radiale fordelingsfunktion (RDF), der gennemsyrer antallet af O- eller H-atomer over alle volumenelementer i en afstand r fra midten af C60, hvor r2 Ľ x2 þ y2 þ z2. Selvom en sådan gennemsnitning øger signal-støj-forholdet, RDF skjuler mange funktioner i det tredimensionelle kort med densitet. Over for disse begrænsninger, vi introducerer den azimutale fordelingsfunktion (ADF), der beregner tæthedskort i tynde sfæriske skaller ved en bestemt r -værdi med hensyn til sfæriske polære koordinater (r, θ, φ). Resultaterne, som præsenterer alle markante tredimensionelle træk ved C60, ses let på papir ved hjælp af Sanson-Flamsteed projektionen (69), der blev brugt af tidlige kartografer. © PNAS, doi:10.1073/pnas.1110626108
(PhysOrg.com) - Vand er vigtigt for mere end dets utallige roller i biologiske, kemisk, geologisk, og andre fysiske processer. Har en præcis beskrivelse af vand struktur er afgørende for at konstruere nøjagtige simuleringer af molekylære begivenheder, herunder proteinfoldning, substratbinding, makromolekylær genkendelse, og kompleks dannelse. Et vigtigt skridt fremad for at skabe en sådan beskrivelse er blevet demonstreret ved Stanford University School of Medicine, hvor forskere opdagede, at polarisering øger den ordnede vandstruktur. Deres fund vil have en betydelig indvirkning på biologiske processer.
Dr. Gaurav Chopra og professor Michael Levitt i Institut for Strukturbiologi brugte molekylære dynamiksimuleringer, der involverede et state-of-the-art Quantum Mechanical Polarizable Force Field (QMPFF3) til at studere hydrering af buckminsterfulleren, den mindste hydrofobe nanosfære, der i vid udstrækning omtales som en buckyball eller C 60 . (Hydrofobe molekyler som fullerener frastødes af vand, og har en tendens til at være både upolære og elektrisk neutrale.)
Der var mange udfordringer at overvinde ved at designe og implementere QMPFF3-baserede molekylære dynamiksimuleringer-især at studere vandmolekylers adfærd ved siden af hydrofobe overflader ved atomdetaljer og subpicosekunders tidsopløsning. ”Det første var behovet for at bruge et passende polariserbart kraftfelt, ”Forklarer Chopra. ”Der findes flere - f.eks. AMOEBA, polariserbare versioner af OPLS, RAV, og CHARMM - men alle disse er empiriske efter at have været parameteriseret til at passe til eksperimentelle data, som de først blev fremlagt af Warshel og Lifson i et papir fra 1968, der diskuterede deres konsekvent kraftfelt . Vi ønskede at bruge et ab initio kraftfelt, der ville være mindre følsomt over for vilkårlig parameterisering. ”Selvom et sådant kraftfelt var blevet udviklet af Algodign, LLC i Moskva, det var ikke tilgængeligt fagligt. Imidlertid, ved at besøge Algodign i Rusland for tre år siden, og med Levitt's intervention, de opnåede akademiske rettigheder.
“Vi startede med at tilpasse QMPFF3 -programmet, AlgoMD, at arbejde på de mange kerner på vores Linux -supercomputer (BioX 2 ) ”Fortsætter Chopra. “Derefter skulle der foretages forskellige tests for at få den korrekte ækvilibreringsprotokol oprettet med det korrekte sæt parametre til normal temperatur- og trykregulering sammen med det mest relevante atomtype-valg til buckyballen, der skal bruges. Det var let at vælge det optimale testsystem, da Levitt-laboratoriet tidligere havde arbejdet på dette molekyle med ikke-polariserbare empiriske kraftfelter. ”
Den sidste udfordring var at finde en metode til at visualisere vandstruktur omkring buckyball. "Den mest populære metode til at studere vandstrukturen omkring et hvilket som helst opløst stof brugte en endimensionel radial fordelingsfunktion, der gennemsnitlige antallet af vand O- og H-atomer i en bestemt afstand for at øge signal-støjforholdet, ”Forklarer Chopra. “Denne endimensionale radiale fordelingsfunktion skjuler mange træk ved det tredimensionelle tæthedskort omkring ethvert vilkårligt formet opløst stof. Står over for disse begrænsninger og for at redegøre for fodboldsymmetrien i C 60 vi introducerede Azimuthal fordelingsfunktion at visualisere O- og H -tætheden fordelt, som den er i koncentriske sfæriske skaller. ”
For at imødekomme disse udfordringer, holdet var afhængigt af en lang historie med beslægtet forskning. ”Siden banebrydende arbejde med det konsekvente kraftfelt udviklet af Shneior Lifson for mere end 50 år siden, modellen af et atom har været en kerne med en delvis ladning. Vi mener, at tiden er kommet til at gå over til en mere realistisk fremstilling af et atom som en kerne og en eksponentielt fordelt nulmasse -elektronsky omkring det. ”Implementeringen af denne repræsentation i QMPFF3 gjorde det muligt at modellere virkningen af polarisering korrekt. We studied the structure of polarized water around polarized Buckminsterfullerene to show that polarization induces a strong hydrophobic effect; this has been under-represented by the limitations due to approximate modeling of atomic interactions in the empirical force fields widely used for the past decades.
The sensitivity of their novel method for detecting surface roughness shows that the hydrophobic effect is much stronger at short- and long-range for QMPFF3 compared to empirical force fields simulations. Af denne grund, QMPFF3 is expected to have a profound effect in understanding key biological processes like protein folding. Using a novel and highly sensitive method to measure surface roughness and detect water ordering, we show that accurate modeling of solute and solvent polarization results in a stronger hydrophobic effect, Chopra summarizes.
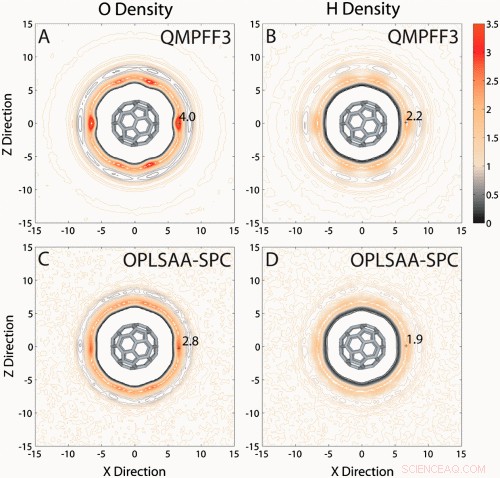
SDFs (QMPFF3 vs. empirical force field). SDFs for water oxygen (O) and hydrogen (H) density around C60 for (A and B) QMPFF3 and (C and D) OPLS-AA with SPC water (OPLSAA-SPC). The orange contours represent higher O and H atom density, and black contours represent lower density than bulk. Both OPLSAA-SPC and QMPFF3 have an excluded volume around C60, a layer of low water density, and well-defined first and second water hydration shells. QMPFF3 water shells around C60 are more structured in radial and azimuthal direction with well-defined peaks; there is no structure apparent in the azimuthal direction for OPLSAA-SPC. The ratio of highest O to H density was 1.4 for OPLS-AA with SPC water and 1.8 for QMPFF3. © PNAS, doi:10.1073/pnas.1110626108
Apart from the azimuthal distribution functions developed to analyze the results, another challenge was to make suitable choices in the simulation protocol to significantly enhance the physical reality of the C 60 water system. The van der Waals equivalent for QMPFF3 is the combination of exchange and dispersion terms in QMPFF3. We used aromatic atom types for C 60 that were reparameterized by simple model correction using coupled-cluster with single and double and perturbative triple excitations data in QMPFF3. We also used long-range dispersion correction terms for total energy and pressure caused by truncation of dispersion forces. Chopra stresses that the study was computationally very intensive, and would not have been possible without the National Science Foundation-supplied BioX 2 supercomputer.
Chopra also points out that while QMPFF3 is one of the best polarizable force fields available today, as it is a general purpose ab initio force field which has been parameterized using only quantum mechanical data to successfully reproduce the experimental data for a large array of chemical compounds in all three phases of matter, it is not perfect. We can reparameterize certain special atom types using a higher level and more accurate quantum mechanical data as well as introduce new atom types for specific applications. The functional form may also need to be modified to further increase the physically realistic representation of the non-bonded parts of the force field currently modeled as dispersion, exchange, electrostatics and induction, he notes. These advances could significantly improve the performance of this state-of-the-art polarizable force field.
På den anden side, Chopra points out, By adapting the QMPFF3 program on GPUs one could significantly increase its computational performance to study much larger systems of interest at biologically relevant timescales. Based on our tests, QMPFF3 is about 10 times slower than the empirical force field simulations to study protein-water systems on commodity clusters. We therefore think it is important to make advances to simultaneously improve the physical reality as well as increase the computational efficiency of the current state-of-the-art polarizable force field.
Chopra sees the teams findings as relevant to a wide range of possible applications. Our work is at the intersection of material science, nanotechnology and fundamental interactions in protein folding. The nature of the hydrophobic effect forms the basis of protein folding simulations and fullerenes are perfect model systems to study the affect of such interactions. I øvrigt, polarization has always been neglected or modeled incorrectly but our results show the importance of polarization resulting in stronger short- and long-range hydrophobic interactions.
Chopra acknowledges that while their findings are not directly applicable to the development of fullerene-based biosensors as such, biosensors are made using water-soluble fullerene derivatives. Having discovered the correct way to include of polarization for your system of interest to make it physically realistic could significantly advance the selection of suitable groups to be attached to fullerenes for many applications, including biosensors, as well as for significantly advancing the process of drug discovery. Our result can be used as a quick way to include the effect of the arrangement of water molecules based on the surface topology of a hydrophobic binding pocket. Generelt, the accurate treatment of polarization to include the affect of solvent in the binding pocket will potentially be useful for advancing computational drug design. Chopra is also very interested to study the effect of polarization on biological systems like proteins in non-homogenous solvent simulations.
Ours is a very general technique and any system can be studied with the simulation and analysis methods of this paper, Chopra concludes. Since QMPFF3 is a general-purpose polarizable force field and, for studying any system, it gives a physically realistic treatment to include polarization, which is essential for any biological system as they are always present in a polar medium like water. Også, our method to study water structure is a significant advance over currently used techniques and should be used to visualize water structure around any arbitrary shaped solute.
Copyright 2011 PhysOrg.com.
Alle rettigheder forbeholdes. Dette materiale må ikke offentliggøres, udsende, omskrevet eller omfordelt helt eller delvist uden udtrykkelig skriftlig tilladelse fra PhysOrg.com.
 Varme artikler
Varme artikler
-
 Låsning af mælkeformel kunne redde liv, siger videnskabsmænd(Phys.org) — En ny undersøgelse af fordøjelsen af mælk kan føre til udviklingen af nye formler til for tidligt fødte børn, vægttabsdrikke og potentielt nye lægemiddelleveringssystemer. Udgivet i
Låsning af mælkeformel kunne redde liv, siger videnskabsmænd(Phys.org) — En ny undersøgelse af fordøjelsen af mælk kan føre til udviklingen af nye formler til for tidligt fødte børn, vægttabsdrikke og potentielt nye lægemiddelleveringssystemer. Udgivet i -
 Et multishot-objektivløst kamera under udvikling kan hjælpe med at diagnosticere sygdomSkematisk layout for et objektivløst kamera. Kredit:Keating/Liu Labs, Penn State En ny type billeddannelse, der ikke kræver en linse og bruger rekonfigurerbare partikelbaserede masker til at tage
Et multishot-objektivløst kamera under udvikling kan hjælpe med at diagnosticere sygdomSkematisk layout for et objektivløst kamera. Kredit:Keating/Liu Labs, Penn State En ny type billeddannelse, der ikke kræver en linse og bruger rekonfigurerbare partikelbaserede masker til at tage -
 De stærkestes overlevelse inden for materialeopdagelseEt selvvalg af peptidnanostrukturer. Kredit:Robert Mart - Cardiff University Forskning ledet af Rein Ulijn, Direktør for CUNY Advanced Science Research Center (ASRC)s Nanoscience Initiative og pro
De stærkestes overlevelse inden for materialeopdagelseEt selvvalg af peptidnanostrukturer. Kredit:Robert Mart - Cardiff University Forskning ledet af Rein Ulijn, Direktør for CUNY Advanced Science Research Center (ASRC)s Nanoscience Initiative og pro -
 Forskere viser vejen frem for at forbedre organiske og molekylære elektroniske enhederDette er et scanningstransmissionselektronmikroskopibillede af en organisk tynd film aflejret på en siliciumnitridmembran. Gule pile angiver gitterorienteringen af hvert krystallinsk domæne. Grønne
Forskere viser vejen frem for at forbedre organiske og molekylære elektroniske enhederDette er et scanningstransmissionselektronmikroskopibillede af en organisk tynd film aflejret på en siliciumnitridmembran. Gule pile angiver gitterorienteringen af hvert krystallinsk domæne. Grønne
- Gecko -adhæsionsteknologi bevæger sig tættere på industrielle anvendelser
- Forskere opdager sikkerhedsfejl i udbredte datalagringsenheder
- Hvordan to vandmolekyler danser sammen
- Teknik tilbyder vej til biofremstilling af medicin under rumflyvninger
- Kan mennesker blive forgiftet ved indirekte udsættelse for polonium-210?
- Vanuatu beordrer evakuering af øen med buldrende vulkan


