De to mødes endelig:Nanotråde og nanorør kombineret for at danne intracellulære bioelektroniske prober
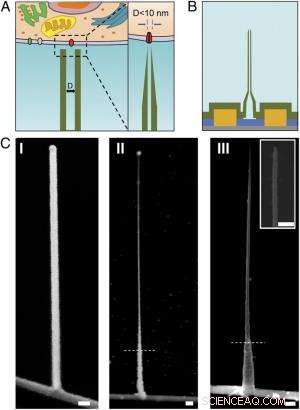
Skema og SEM-billeder af den ultralille BIT-FET. ( EN ) Skematisk illustration af en intracellulær bioelektronisk probe. ( Venstre ) Generelt skema for en probe til intracellulær elektrofysiologisk registrering. ( Ret ) Et forstørret billede af spidsen af en sub-10-nm bioelektronisk sonde og dens relaterede størrelse til en enkelt ionkanal. ( B ) Skematisk struktur af den ultralille BIT-FET. Grøn, gul, blå, og grå farver repræsenterer SiO 2 lag, metalkontakt, SiNW, og siliciumnitridsubstrat, henholdsvis. ( C ) SEM-billeder af den ultralille BITFET ved forskellige fremstillingstrin. En GeNW-gren blev først dyrket oven på SiNW ( jeg ), efterfulgt af et efterfølgende H 2 O 2 ætsning af den øverste del af GeNW for at krympe dens diameter ned til sub-10 nm regime ( II ). En sidste visning af en ultralille BIT-FET med nanorør ID ~8 nm, og SiO 2 vægtykkelse ~10 nm er præsenteret i ( III ). Indsat af III er nærbilledet af spidsen af den ultralille SiO 2 nanorør. Hvide stiplede linjer ind II og III angive det punkt, under hvilket GeNW og SiO 2 er beskyttet af fotoresist under H 2 O 2 og BHF ætsning, henholdsvis. Alle skala søjler:100 nm. Kredit:Copyright © PNAS, doi:10.1073/pnas.1323389111
(Phys.org) —Miniaturiserede bioelektroniske prober står til at transformere biologi og medicin ved at tillade måling af intracellulære komponenter in vivo . For nylig, videnskabsmænd ved Harvard University og Peking University designet, fremstillet og demonstreret bioelektroniske prober så små som 5 nanometer ved hjælp af en unik tredimensionel nanotråd-nanorør-heterostruktur. (En heterostruktur kombinerer flere heterojunctions – grænseflader mellem to lag eller områder af uens krystallinske halvledere – i en enkelt enhed.) Gennem eksperimentelle målinger og numeriske simuleringer, forskerne viste, at disse enheder har tilstrækkelig tidsopløsning til at optage de hurtigste elektriske signaler i neuroner og andre celler, med integration i større chip-arrays, der potentielt giver ultra-høj opløsning kortlægning af aktivitet i neurale netværk og andre biocellulære systemer.
Prof. Xiaojie Duan diskuterede papiret, at hun, Kandidatforsker Tian-Ming Fu, Prof. Charles M. Lieber og deres medforfattere udgivet i Proceedings of the National Academy of Sciences . Hun påpeger først, at nanorørsonder og deres heterojunction med silicium nanowire field-effect transistorer (SiNW FET'er) bliver mekanisk mindre stabile, når diameteren reduceres. "Når nanorøret bliver mindre og mindre, Duan fortæller Phys.org, "det bliver nemmere at bryde nanorøret i forbindelsesområdet med SiNW. Ved anvendelse af sonden til intracellulær bioelektronisk detektion, der vil være forskellige kræfter, såsom kapillærkraften fra væsken, samt interaktion mellem proben og cellemembranen. Disse kræfter kan bryde sonden, hvis vi har et svagt kryds mellem den og SiNW."
Et andet problem er, at den elektriske følsomhed også reduceres, når nanorørets diameter falder, fordi nanorørets indre diameter (ID) definerer det effektive enhedsportsområde. "I optagelsen af intracellulært transmembranpotentiale ved hjælp af vores sonde, Duan forklarer, "cytosol fylder nanorøret og fungerer som gateelektroden for den underliggende SiNW FET." Cytosol (også kaldet intracellulær væske eller cytoplasmatisk matrix ) er den væske, der findes inde i celler, med undtagelse af organeller og andre cytoplasmatiske komponenter. "Cytosolpotentialeændringen modulerer bærertætheden af SiNW FET, og derved ændre dens ledningsevne, " Duan fortsætter. "Sådan fungerer vores sonde til bioelektronikoptagelse." Kontaktområdet mellem cytosolen og SiNW - defineret af den indre diameter af nanorøret - bestemmer effektiviteten af konduktansmodulationen. Med andre ord, hvis nanorørets indre diameter er for lille, SiNW FET-portområdet vil også være for lille.
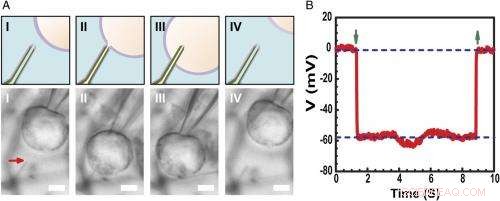
Optagelse af intracellulær hvilemembranpotentiale. ( EN ) Skema (øvre) og differentiel interferens kontrast optiske mikroskopi billeder ( Nederste ) af en HL-1-celle manipuleret af en glasmikropipette til at nærme sig ( jeg ), kontakt ( II ), trænge ind ( III ), og træk tilbage ( IV ) fra en phospholipid-modificeret ultralille BITFET-probe. Den røde pil angiver positionen af den ultralille nanorørspids. Fordi ren SiO 2 nanorøret er optisk gennemsigtigt, GeNW-skabelonen for denne enhed blev ikke ætset til billeddannelse. Målestok:2 μm. ( B ) Repræsentative elektriske optagelser resultater fra en ∼10 nm ID ultralille BIT-FET-enhed; I dette tilfælde, GeNW blev ætset for at give den ultralille SiO 2 nanorør. Grønne pile, der peger ned og op, markerer begyndelsen af cellepenetration og tilbagetrækning, henholdsvis. De øvre og nedre vandrette stiplede linjer angiver de ekstracellulære og intracellulære potentialer. Kvasistatiske vand-gate-målinger foretaget før/efter cellemålinger viser <2 % ændring i enhedens konduktans og følsomhed. Kredit:Copyright © PNAS, doi:10.1073/pnas.1323389111
Forskerne blev også præsenteret for det faktum, at højfrekvent dynamisk respons kan nedbrydes med faldende nanorørs indre diameter på grund af stigende opløsningsmodstand i nanorøret. "Når nanorørets indre diameter falder, Duan forklarer, "modstanden af opløsningen inde i nanorøret vil stige, på grund af faldet i opløsningslederens tværsnit." hastigheden, hvormed den underliggende SiNW FET kan reagere på et signal, bestemmes af produktet RC af kapacitansen og modstanden af opløsningslederen inde i nanorøret - så hvis nanorøret bliver mindre, modstanden bliver større. Det betyder, at SiNW FET skal bruge mere tid til at reagere – og hvis signalændringen er for hurtig, sonden vil ikke være i stand til pålideligt at registrere det. "Det er, hvad vi mener med 'højfrekvent dynamisk respons vil forringes med faldende nanorørs indre diameter, Duan tilføjer, "Imidlertid, vi fandt det til vores sonde, selvom vi mindsker nanorørets indre diameter til så lille som 5 nm, sonden er stadig i stand til at optage et 3 kHz-signal trofast – en båndbredde, der i de fleste tilfælde er tilstrækkelig til at optage neurale og hjertesignaler."
Forskerne blev også konfronteret med behovet for at bruge aktive halvleder-nanowire-felteffekttransistordetektorer for at overvinde begrænsningerne ved reduktion af sondestørrelse. "Ordet aktiv" sammenlignes med den "passive" karakter af optagelse med metalelektroder, " påpeger Duan. "Til metalelektrodeoptagelse, a portion of the transmembrane potential V m will be dropped or lost at the electrode/electrolyte interface, so the signal recorded will be smaller than the real transmembrane potential." When the size of the metal electrode is reduced, the impedance value at the electrode/electrolyte will increase – and at some point, this impedance will get so large that the recorded signal will be obscured by noise. Imidlertid, for the FET recording, the cytosol potential change is reflected by the semiconductor channel conductance change, which is independent of the probe/electrolyte interface impedance. "Since decrease in probe size will therefore ikke affect the signal amplitude, " Duan adds, "using the FET to sense potential is a very effective way to overcome the limitations of probe-size reduction."
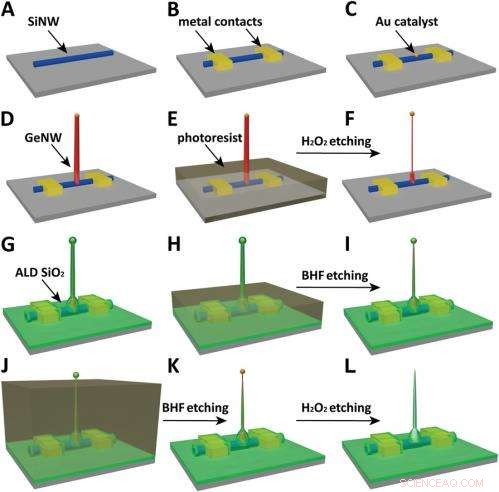
Schematics of the fabrication flow for the ultrasmall BIT-FET. ( EN ) SiNWs (blue) are dispersed on substrate (solid gray). ( B ) S/D contacts are defined by EBL followed by thermal evaporation. ( C ) Au nanodots are defined on SiNWs between S/D using EBL and thermal evaporation. ( D ) GeNWs (red) are grown on top of the SiNWs through nanocluster-catalyzed CVD process. ( E ) A thin layer of photoresist (transparent gray) is spin coated on the chip to protect the lower GeNW part. ( F ) The resulting H 2 O 2 -etched GeNWs following photoresist liftoff. Only the GeNW above the photoresist in E is thinned by etching in H 2 O 2 . (G) SiO 2 is conformally deposited over the entire chip by ALD. ( H ) A thin layer of photoresist (transparent gray) is spin coated to protect the lower region of chip. ( jeg ) The resulting BHF etched structures following liftoff. The region of SiO 2 above the photoresist layer in H is etched to ca.10-nm thickness. ( J ) Photoresist with thickness smaller than the GeNW heights is deposited. ( K ) The resulting structure following BHF etching of SiO2, which exposes the tips of the GeNWs. Isotopic BHF etching yields a small taper with thinner SiO 2 at the topmost part of the structure. (L) The GeNW is removed by H 2 O 2 etching to form an ultrasmall nanotube connected to the bottom SiNW FET. Copyright © PNAS, doi:10.1073/pnas.1323389111
Another critical challenge was synthetically integrating nanotubes and nanowires. "The nanotube is made of silicon oxide and the nanowire is made of silicon, " Du an notes. "For cell recording, the nanotube needs to be built vertically onto the nanowires – meaning that a three-dimensional heterostructure is needed." While the heterostructure could be fabricated in various ways, not all of them provided the required controllability at the probe scale. Derfor, the researchers grew a germanium nanowire (GeNW) on top of the silicon nanowires, with the GeNW acting as a template for the nanotube. "Depositing SiO 2 on the GeNW/SiNW heterostructure, then selectively removing the core GeNW, resulted in the desired nanotube/SiNW structure, " notes Duan. "Using this method, the nanotube inner diameter can be controlled by the GeNW diameter, the outer diameter can be controlled by the SiO 2 thickness, and the nanotube length can be defined by the GeNW growth time. This gives us complete and easily implemented control over the probe's dimensions."
Endelig, the scientists had to investigate and model the bandwidth effect of phospholipid coatings, which are important for intracellular recording. "We use phospholipid coating to assist the nanotube probe to penetrate the cell membrane, " Duan notes. "Since the bandwidth of our probe is important for the probe to be able to record fast neural or cardiac signal, we need to make sure this phospholipid modification will not overly affect the bandwidth." Because the phospholipid layer will decrease the cross section of the solution conductor inside the nanotube, it impacts bandwidth in two ways by changing the capacitance and resistance of the solution inside the nanotube. (The phospholipid-modified probe bandwidth is easily determined by applying a fast artificial signal to the solution, and then recording how the conductance of the device changes over time. This provides the time the device needs to respond to this signal and thus the device's bandwidth.) "Therefore, " Duan explains, "if the nanotube is large, this thin phospholipid layer will not cause too much of a difference. Imidlertid, for our probe, the sub-10 nm nanotube size is almost the same scale as the lipid layer – so we have to carefully examine how it affects probe bandwidth, both experimentally and theoretically."
The scientists addressed these myriad challenges in designing, fremstilling, and demonstrating the probe with three key innovations. "The first is the use of FET as potential sensing element, which in principle enables us to overcome the size limit on the probe." Duan explains. "However, FETs have conventionally existed in a linear geometry with connections that preclude access to the inside of cells." The second innovation was the solution to this problem – namely, the design of a vertical SiO 2 nanotube on top of the nanoscale FET, which allowed them to introduce cytosol into the cell without having to insert the FET channel inside the cell, which would be more invasive. The third key is the design of a relatively large nanotube base with a much sharper nanotube tip, resulting in a sub-10 nm probe without sacrificing its mechanical strength and electrical sensitivity.
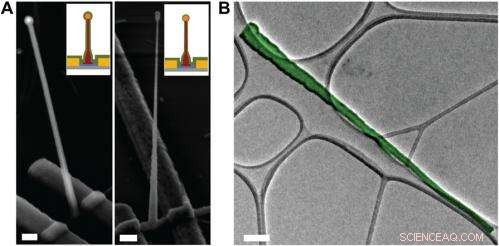
Electron microscopy characterization of the ultrasmall BIT-FET. ( EN ) Representative SEM (Zeiss Ultra Plus field-emission SEM) images of intermediate fabrication steps of the ultrasmall BIT-FET. ( Left ) Device after 30-nm ALD coating of SiO 2 . ( Right ) Device after first step of selective BHF etching of the upper80% portion of the SiO 2 to ca. 10 nm (Fig. S1 H og jeg ). White dashed lines in jeg og II indicate the point below which the SiO 2 is protected by photoresist during BHF etching. Scale bars:200 nm. ( B ) False-colored transmission electron microscopy (JEOL 2100 TEM) image of an ultrasmall nanotube. This tube was fabricated following the same procedure as described in SI Text , and deposited onto lacey carbon grids (Ted Pella) from ethanol suspension. It has a tip ID ∼7 nm and bottom ID ∼80 nm. False color is used here to distinguish the SiO 2 nanotube from background amorphous carbon. Scale bar:50 nm. Kredit:Copyright © PNAS, doi:10.1073/pnas.1323389111
An important aspect of the study was ensuring that the bioelectronic devices had sufficient time resolution to record the fastest electrical signals in neurons and other cells. "The signals in neural system are normally in the millisecond scale, " Duan points out. "That means that to reliably record these signals, the recording device needs to have a bandwidth measure in kilohertz." While the probe's bandwidth decreases with the decrease of nanotube diameter, the scientists found that even for probes with inner diameters as small as 5 nm, the bandwidth is still around 3 kHz (a time resolution ~ 0.3 ms) in physiological solution. "This means that our probes have sufficient time resolution to record the fastest electrical signals in neurons and other cells, " Duan adds.
I øvrigt, Duan points out, the scientists found that measuring the cell transmembrane resting potential with these ultrasmall bioelectronic devices demonstrates the capability for intracellular electrophysiology studies. "When we measured the transmembrane resting potential of HL-1 cell with our new probes, we found that with the phospholipid modification, the nanotube can easily and reliably penetrate the cell membrane, allowing the FET to record the intracellular transmembrane potential at full amplitude." After retracting the nanotube from the cell, the recorded potential can immediately revert to the extracellular potential. "Reliable cell membrane penetration and stable recording of intracellular transmembrane potential prove the capability of our probes for intracellular electrophysiology studies, " notes Duan.
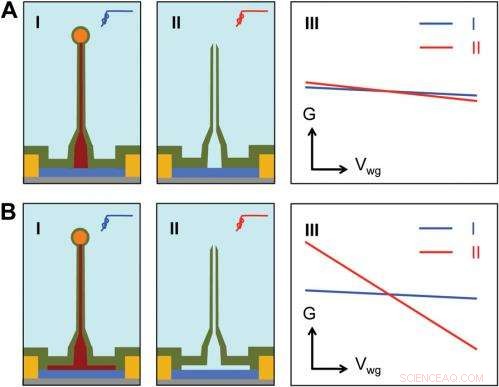
Sensitivity of different device structures. ( EN , B ) Schematics of the ultrasmall BIT-FET without and with Ge overcoating on the SiNW, henholdsvis. jeg og II correspond to the BIT-FET devices before and after Ge core etching. III show schematically typical conductance ( G ) vs. water-gate (V wg ) measurements from these distinct structures. Kredit:Copyright © PNAS, doi:10.1073/pnas.1323389111
Bevæger sig fremad, says Duan, the researchers' are planning to scale up their work to integrate the probes into high-density, large-scale array for large-scale mapping of neural activities; use the probes to record neural signals from small subcellular structures/organelles; and investigate other applications in which the probes will provide substantially greater spatial resolution and minimal invasiveness than other techniques.
Ud over, the scientists might consider developing other innovations. "For eksempel, " Duan illustrates, "a major challenge in using our ultra-small probes for recording from small subcellular structures is to accurately position them with respect to the subcellular structures of interest. We're looking at either labeling our probe with fluorescence dye – or other biocompatible materials – to mark the nanotube at high resolution, or using specific targeting in which the probe's biochemical surface groups define the specific cell location being studied."
Duan sees other areas of research that might benefit from their study, inklusive:
- Neuroscience:Since recording of intracellular action potential and other low frequency transmembrane potential signal is important to study the neural network.
- Medicine:By studying how the drugs affect the recorded intracellular action potentials or other transmembrane potentials, the probes can provide an attractive technique for parallel or high-throughput screening of drugs targeting the ion channels.
- Biophysics:The process of cell membrane penetration by the phospholipid-modified nanotube can serve as a useful platform for studying the cell membrane's fusion-related biological processes.
© 2014 Phys.org. Alle rettigheder forbeholdes.
Sidste artikelDiamane:Diamantfilm muligt uden tryk
Næste artikelMønstre af partikler genereret af overfladeladninger
 Varme artikler
Varme artikler
-
 Forskere bruger guld nanorodspredning til at identificere immunsystemets dræber og frelserKredit:CC0 Public Domain Ethvert biologisk system er naturligvis udstyret med en forsvarsmekanisme til at beskytte mod unormale ændringer forårsaget af enten lokale, miljø, eller biokemisk ændring
Forskere bruger guld nanorodspredning til at identificere immunsystemets dræber og frelserKredit:CC0 Public Domain Ethvert biologisk system er naturligvis udstyret med en forsvarsmekanisme til at beskytte mod unormale ændringer forårsaget af enten lokale, miljø, eller biokemisk ændring -
 Sådan bygger du donuts med legoklodserDette viser AFM-tapping-amplitudesignal fra en diblokring i de sene stadier af udglødning. En Plateau-Rayleigh-lignende ustabilitet har udviklet sig, og ringen viser dannelsen af fire distinkte dråb
Sådan bygger du donuts med legoklodserDette viser AFM-tapping-amplitudesignal fra en diblokring i de sene stadier af udglødning. En Plateau-Rayleigh-lignende ustabilitet har udviklet sig, og ringen viser dannelsen af fire distinkte dråb -
 Gennembrud inden for molekylær elektronik baner vejen for DNA-baserede computerkredsløb i fremtide…Baner vejen for en ny generation af DNA-baserede computerkredsløb:Prof. Danny Porath, fra Hebraisk Universitets Institut for Kemi og Center for Nanovidenskab og Nanoteknologi. Kredit:Hebrew University
Gennembrud inden for molekylær elektronik baner vejen for DNA-baserede computerkredsløb i fremtide…Baner vejen for en ny generation af DNA-baserede computerkredsløb:Prof. Danny Porath, fra Hebraisk Universitets Institut for Kemi og Center for Nanovidenskab og Nanoteknologi. Kredit:Hebrew University -
 Løft af låget på silikonebatterier(Phys.org) — At løse mysteriet om, hvad der sker inde i batterier, når silicium kommer i kontakt med lithium, kan fremskynde kommercialiseringen af næste generations højkapacitetsbatterier, til brug
Løft af låget på silikonebatterier(Phys.org) — At løse mysteriet om, hvad der sker inde i batterier, når silicium kommer i kontakt med lithium, kan fremskynde kommercialiseringen af næste generations højkapacitetsbatterier, til brug
- Tidlige livsstadier af egern
- Forældre inden for STEM-områder øger pigernes deltagelse i naturvidenskabelige grader
- Cassini finder The Big Empty tæt på Saturn
- Subsiderende luftpakker er blandt årsagerne til varme perioder
- Trumps idé om at regulere Google uudgrundelig
- Forskeren finder forskelle, men ingen generelle tendenser, når potentielle forurenende stoffer bind…


