Bestemmelse af topografiske strålingsdosisprofiler ved hjælp af gel nanosensorer
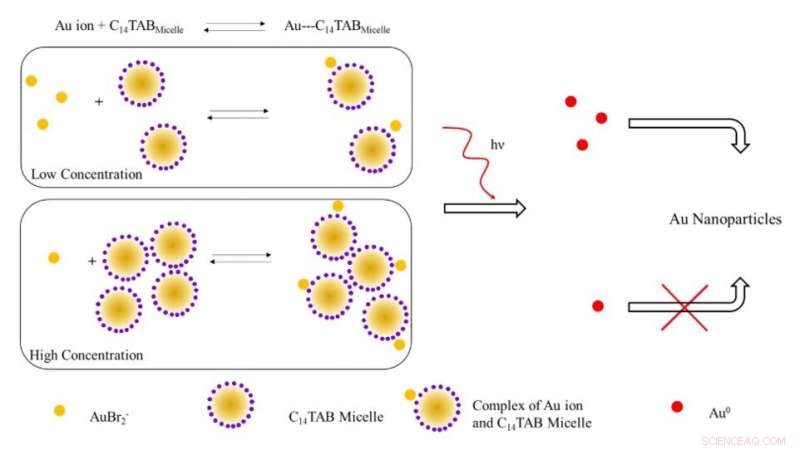
Skematisk illustration af den foreslåede mekanisme til dannelse af guld nanopartikler ved bestråling med ioniserende stråling. Ved lave koncentrationer af overfladeaktive stoffer, de fleste guldioner (AuBr2- /Au1+) er sandsynligvis frie i opløsning (ubundet til miceller). Med stigende koncentration af overfladeaktivt stof, ligevægten skifter til højre med et fald i frie guldioner. Ved bestråling, antallet af Au^0-atomer dannet på grund af reduktion ved lav overfladeaktivt stofkoncentration er højere på grund af tilstedeværelsen af et højere antal frie guldioner i modsætning til systemet ved høj overfladeaktivt stofkoncentration. Det højere antal frie guldatomer fører til et øget udbytte af guldnanopartikler på grund af overfladeassisteret reduktion med uomsatte guldioner. Kredit:Science Advances, doi:10.1126/sciadv.aaw8704.
Den rutinemæssige måling af strålingsdoser kan være klinisk udfordrende på grund af begrænsninger med konventionelle dosimetre, der bruges til at måle dosisoptagelsen af ekstern ioniserende stråling. I en ny undersøgelse, Karthik Pushpavanam og et tværfagligt team af forskere i afdelingerne for Kemiteknik, Molekylær Videnskab, Banner MD Anderson Cancer Center og Arizona Veterinary Oncology i USA har beskrevet en ny gel-baseret nanosensor. Teknologien tillader kolorimetrisk påvisning og kvantificering af topografiske stråledosisprofiler under strålebehandling.
Ved udsættelse for ioniserende stråling, forskerne omdannede guldioner i gelen til guldnanopartikler (AuNP'er) ledsaget af en visuel ændring i gelens farve på grund af plasmoniske egenskaber. De brugte intensiteten af farve dannet i gelen som en kvantitativ reporter til ioniserende stråling og brugte først gel nanosensoren til at detektere komplekse topografiske dosismønstre efter administration til antropomorfe fantommodeller efterfulgt af applikationer med levende hundepatienter, der gennemgår klinisk strålebehandling. Den lette fremstilling, operation, hurtig aflæsning, kolorimetrisk detektion og relativt lave omkostninger ved teknologien indebar translationel potentiale for topografisk dosiskortlægning under kliniske strålebehandlingsapplikationer. Forskningsarbejdet er nu offentliggjort på Videnskabens fremskridt .
Fremskridt inden for stråleterapi har ført til bemærkelsesværdig sofistikeret og avanceret planlægningssoftware til at levere høje konforme stråledoser til patienter for forbedret livskvalitet efter behandling. Under strålebehandling, en høj dosis afgives typisk til en måltumor, mens den strålingsdosis, der leveres til omgivende væv, minimeres. Under palliativ behandling administreres patienter med større fraktionerede doser for at afslutte behandlingen inden for en kort tidsramme. Imidlertid, softwarefejl under sådanne procedurer kan føre til overdosering og efterfølgende morbiditet.
For at minimere utilsigtet overeksponering, forskere søger uafhængigt at verificere dosis af stråling leveret ved eller nær målvævet for avanceret patientsikkerhed. Teknisk set, både molekylære og nanosensorer kan overvinde grænser, der findes i konventionelle systemer, for at danne praktiske alternativer som nemme sensorer. Imidlertid, deres eksisterende grænser bør adresseres og afhjælpes for at udvikle robuste og effektive sensorer, der kvantitativt og kvalitativt bestemmer de topografiske dosisprofiler under klinisk strålebehandling.

Digitale billeder og UV-synlige spektre af forskellige gel nanosensor formuleringer udsat for terapeutiske doser af røntgenstråler (A) Billeder af gel nanosensorer fremstillet i 24-brønds cellekulturplader og indeholdende forskellige koncentrationer af C14TAB (24,5 til 73,5 mM) ved eksponering for forskellige doser af ioniserende stråling (0- til 10-Gy røntgenstråler); Na2S ventetiden var 5 minutter efter bestråling, og inkubationstiden var 10 min. Billeder blev erhvervet 1 time efter bestråling. En synlig stigning i intensiteten i den rødbrune farve observeres med stigende doser af ioniserende stråling for de fleste C14TAB-koncentrationer, der anvendes under udvikling af gelsensorer. (B til F) Absorbansspektre (300 til 990 nm) af de samme gel nanosensorer indeholdende (B) 24,5 mM, (C) 31 mM, (D) 37 mM, (E) 49 mM, og (F) 73,5 mM bestrålet under anvendelse af forskellige strålingsdoser. Karakteristiske absorbanstoppe mellem 500 og 600 nm bølgelængder er tegn på guld nanopartikler dannet i gelerne. De tilsvarende stråledoser er nævnt i forklaringen med stigende stråledosis (top til bund). A.U., vilkårlige enheder. Fotokredit:Sahil Inamdar, Arizona State University. Kredit:Science Advances, doi:10.1126/sciadv.aaw8704
Siden guld nanopartikler (AuNP'er) har unikke fysiske og kemiske egenskaber, der giver en fremragende platform til at udvikle sensorer. Pushpavanam et al. konstrueret en kolorimetrisk sensor, hvor ioniserende stråling forårsagede AuNP-dannelse fra farveløse saltprækursorer. Dannelsen af en gel-baseret nanosensor kan tillade nem håndtering og anvendelse under klinisk strålebehandling.
I nærværende arbejde, holdet demonstrerede kolorimetrisk detektion og kvantificering af dosisfordelingsprofiler ved hjælp af en gel nanosensor til topografisk kortlægning af strålingsdoser langs vævsoverflader. Under prækliniske evalueringer, holdet administrerede gel nanosensor-teknologien til levende hundepatienter, der gennemgår strålebehandling. I alt, resultaterne indikerede omfanget af teknologien til klinisk translation hos humane patienter og kapaciteten til at bestemme topografiske doser for at planlægge behandlinger og verificere doser under cancerstrålebehandling.
Under forsøgene, omdannelsen af guldioner til nanopartikler blev ledsaget af en rødbrun farveudvikling i det bestrålede område af gel-nanosensoren. Mens guld eksisterer i en trivalent tilstand generelt (AuCl 4 - ) den kan reduceres til en metastabil +1 valenstilstand (AuBr 2 - ) ved stuetemperatur under anvendelse af ascorbinsyre (C-vitamin). Bestrålingen af geler indeholdende terapeutiske niveauer af stråling stimulerede radiolyse eller spaltningen af vandmolekyler i meget reaktive frie radikaler. De radiolyse-genererede hydratiserede elektroner reducerede igen monovalent guld til at danne guldatomer i dets nulvalente tilstand (Au 0 ), der nukleerede og modnede til rødbrune AuNP'er. Intensiteten varierede med strålingsdosis, og holdet brugte rækken af lineære responser til at kalibrere gel nanosensoren. Ud fra dette princip, Pushpavanam et al. bestemte responsen af de fuldt bestrålede geler til at kalibrere absorbans med strålingsdosis.
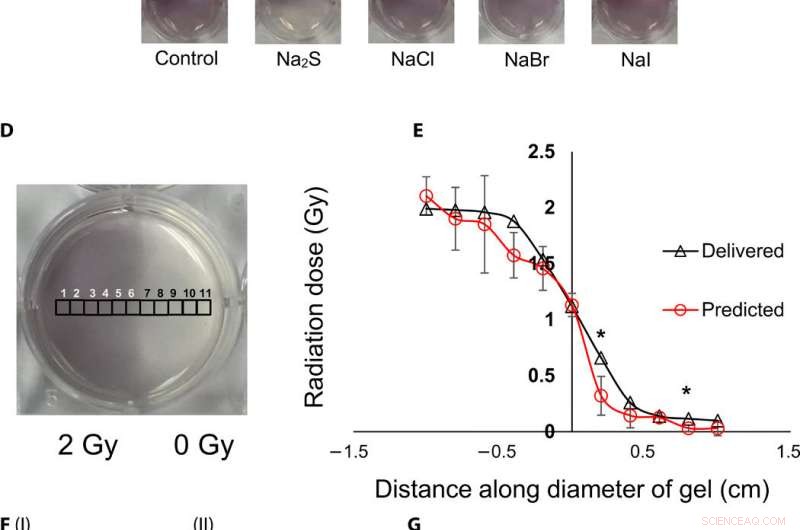
Topografisk visualisering og kvantificering af strålingsdoser ved hjælp af gel nanosensorer. (A) Gel nanosensor (venstre) før bestråling, (midterste) øverste halvdel bestrålet med 4 Gy og billede erhvervet 2 minutter efter bestråling, og (højre) billede opnået 1 time efter bestråling. A visible increase in color intensity in the nonirradiated lower half indicates bleed over of color and loss of topographical information. (B) I:1.5% (w/v) agarose gel (left) 2 min after irradiation and (right) 1 hour after irradiation; II:2% (w/v) agarose gel (left) 2 min after irradiation and (right) 1 hour after irradiation indicates that the increase in agarose weight percentage does not preserve topographical dose information. (C) Gel nanosensor incubated with 5 mM sodium sulfide (Na2S) and various sodium halides with a wait time of 10 min and incubation time of 10 min; images were acquired after 1 hour. No loss of topographical information is observed upon incubation with sodium sulfide. All gels were fabricated in 24-well plates. (D) Colorimetric response of the gel nanosenor irradiated on one-half with a 2-Gy x-ray dose. A visible appearance of maroon color in the irradiated region illustrates the ability of the gel nanosensor to visualize topographical dose profiles. Each black square box (labeled 1 to 11) on the gel nanosensor corresponds to a grid of size ≈2 × 2 mm, whose absorbance at 540 nm is determined. Grids starting from 1 to 5 are regions exposed to ionizing radiation, 6 is the grid at the edge of the irradiation field, and grids from 7 to 11 are regions outside the field of irradiation. (E) Dose fall-off profile for the gel nanosensor irradiated by 2 Gy on one-half. The delivered and predicted radiation doses are comparable, which indicates the efficacy of the gel nanosensor in visualizing and retaining topographical information. In all cases, Na2S was added for 10-min incubation time after a wait time of 30 min. Radiation doses predicted by the gel nanosensor as compared with the delivered radiation dose as obtained from the treatment planning system. Asterisks indicate statistically significant differences (P <0.05) between the delivered dose and the dose predicted by the gel nanosensor (n =3 independent experiments). (F) Representative image of a petri dish containing the gel nanosensor formulation (≈3 mm thick and ≈10 cm diameter) irradiated with a 1 cm × 1 cm square field of x-ray radiation. From the left, each square indicates increasing radiation dose from (I) 0.5 Gy (red box), 1 Gy, and 1.5 Gy; (II) 2, 2.5, 3, and 3.5 Gy; and (III) 4, 4.5, and 5 Gy; the black box in image (II) shows 0 Gy. (G) Visualization of a complex topographical dose pattern (ASU letters) generated using a 2-Gy x-ray dose. The petri dish has a diameter of ≈10 cm. In (F) and (G), the gel nanosensors contain 24.5 mM C14TAB, and Na2S was added after a wait time of 30 min and incubation time of 10 min; a representative image from three independent experiments is shown. Photo credit:Sahil Inamdar, Arizona State University. Kredit:Science Advances, doi:10.1126/sciadv.aaw8704.
To determine intensity of the color and dose delivered within gels after irradiation, the researchers used absorbance spectroscopy and observed a decrease in the spectral profile width, with increasing radiation dose for decreased polydispersity (ratio of the percentage of the standard deviation to the average value) of the nanoparticles. The peak absorbance intensity increased with increasing radiation dose to corroborate the observed increase in color intensity.
To understand the gel nanosensor's ability to detect topographical distribution of the radiation dose, the scientists irradiated half of the gel nanosensor with a 4 gray (Gy) dose. The maroon-color only appeared in the irradiated area confirming AuNP formation, but after one hour of exposure, the color bled into the irradiated region showing loss of topographic information in the gel with time. The team observed the phenomenon to arise from reaction-controlled conditions and not based on the gel composition. By incubating the gel with sodium sulfide (Na 2 S) for 10 minutes, they suppressed the color bleed-over and reasoned that to the ability to quench unreacted gold ions in the nonirradiated region and preserve dose information accurately for dose visualization and dosimetry. The scientists adopted the sensor for wide dose ranges by modulating the time of Na 2 S addition; to achieve a level of flexibility hitherto unavailable in clinical dose detection systems.
The research team then used the gel nanosensor to visualize diverse topographical radiation patterns, where the intensity of the color increased with increasing dose while preserving topographical integrity. As proof of concept, they showed the gel nanosensor's ability to detect complex radiation patterns with a model dose patterned to form "ASU" (after Arizona State University). Then using transmission electron microscopy (TEM), the scientists characterized the generated gold nanoparticles as a function of dose to observe reduced average nanoparticle diameter and polydispersity at higher doses of radiation. They followed this with energy dispersive X-ray spectroscopy (EDS) to detect higher yields of AuNPs in the irradiated regions of the gel nanosensor as expected.
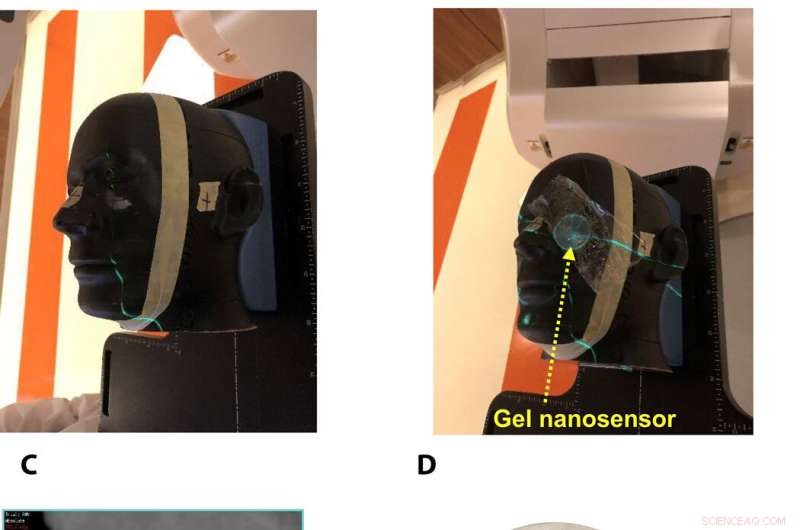
Gel nanosensor enabled topographical detection and quantification of clinical radiation doses in anthropomorphic head and neck phantoms. (A) Anthropomorphic head and neck phantom treated with an irregularly shaped x-ray radiation field below the left eye. (B) Image of the gel nanosensor positioned on the anthropomorphic phantom in the radiation field mimicking a conventional radiotherapy session. (C) Axial view of the treatment planning image along the central axis of the radiation beam representing an irregularly shaped radiation field used to deliver a complex radiation pattern under the eye of the phantom. The core of the crescent-shaped treatment region receives a radiation dose of 2.3 Gy (highlighted in red), and regions receiving lower doses are highlighted with different colors going outward (from green to light pink). (D) Visual image of the dose pattern on the gel nanosensor formed after delivery of 2.3 Gy. Only the irradiated region develops a maroon color, while the nonirradiated region remains colorless. (E) Expected topographical dose “heat map” profile of the radiation dose delivered to the gel placed in the phantom. The expected profile is generated from the treatment plan in the dose delivery system. In these figures, red and blue colors indicate higher and lower radiation doses, henholdsvis. (F) Topographical doses predicted by the irradiated gel nanosensor. Absorbance values of ≈2 mm × 2 mm grids were quantified using a calibration curve to generate the topographical dose profile. The anticipated dose received by the core of the crescent-shaped profile (2.3 Gy) is comparable to the dose profile predicted by the gel nanosensor (2.3 Gy), which demonstrates the capability of the gel nanosensor to qualitatively and quantitatively detect complex topographical dose profiles. Photo credit:Sahil Inamdar, Arizona State University. Kredit:Science Advances, doi:10.1126/sciadv.aaw8704.
To investigate translational potential of the gel nanosensor and predict topographical profiles of radiation, Pushpavanam et al. first used a head and neck phantom model. They delivered an irregular crescent-shaped radiation dose near the eye to mimic clinically challenging administration modes of radiotherapy close to critical structures such as the eye during skin cancer treatment. The dose profile delivered using the treatment planning system was in excellent agreement with the predictions of the gel nanosensor. Indicating its capability to detect and predict complex radiation patterns similar to those used in clinical human radiotherapy.
During preclinical studies, the research team used two canine models undergoing radiotherapy to investigate the efficiency of gel nanosensors as independent, nanoscale radiation dosimeters for the first time and compared the efficiency with conventional clinical radiochromic films. On completion of the treatment, Pushpavanam et al. observed maroon color formation in one-half of the gel, whereas the non-irradiated region remained colorless. They showed predictions of the gel nanosensor in the irradiated region to agree excellently with the treatment planning system and the radiochromic film. The gel nanosensor also predicted for the region external to the irradiation to receive minimal radiation and their topographical dose profiles as well. The performance was comparable to clinical radiochromic films but with faster than conventional wait times (typically> 24 hours) to obtain the results. The scientists demonstrated the simplicity of fabrication, operation, readout time and cost effectiveness ($ 0.50 per gel material only) of the frugal invention. They maintained the response of the gel nanosensor for at least seven days to indicate long-term retrieval of dosing data unlike with fluorescence-based dosimeters with readouts that lasts mere minutes.
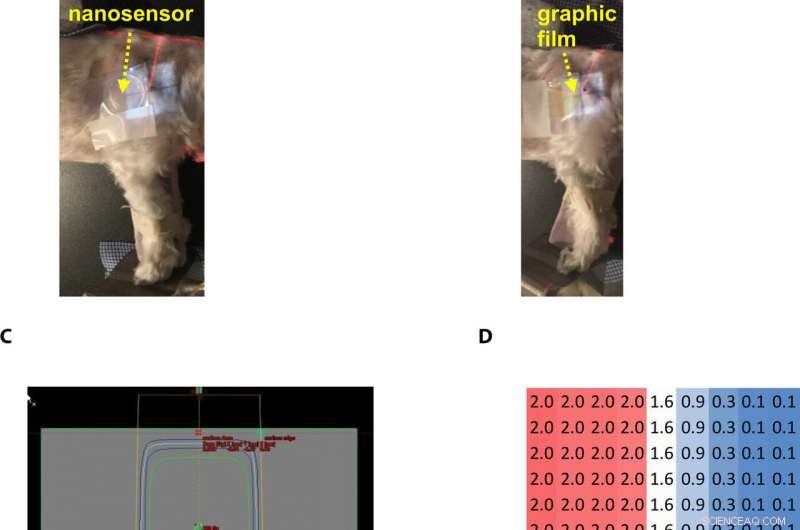
Gel nanosensor enabled topographical detection and quantification of radiation delivered to canine patient A undergoing clinical radiotherapy. Representative image of (A) half of the gel nanosensor and (B) half of the radiographic film positioned in the radiation field delivered to canine patient A. (C) Treatment planning software depicting the delivery of a 2-Gy dose delivered to the surface of patient A (neon green edge along the rectangular gray box indicates the region receiving the 2-Gy dose). (D) The irradiated region received a dose of 2 Gy (highlighted in red squares), with irradiation dose dropping to a minimal radiation 0.1 Gy (highlighted in blue squares) outside the field of irradiation. A color change is visible in both the (E) gel nanosensor whose color changes to maroon and (F) radiographic film whose color changes to dark green after irradiation. The predicted dose map in the gel nanosensor (Na2S addition wait time of 30 min and incubation time of 10 min) and radiographic film are shown below each corresponding sensor. Similarity in the dose profiles indicates the efficacy of the gel nanosensor for clinical dosimetry. The time for readout of the gel nanosensor was 1 hour after irradiation, while the radiochromic film required>24 hours of developing time before readout. All experiments were carried out three independent times. Photo credit:Sahil Inamdar, Arizona State University. Kredit:Science Advances, doi:10.1126/sciadv.aaw8704.
På denne måde Karthik Pushpavanam and colleagues developed the first colorimetric gel nanosensor as a nanoscale dosimeter to detect and distinguish regions exposed to irradiation. They optimized the platform with a chemical quenching agent (Na 2 S) to accurately reveal topographical dose distribution during clinical radiotherapy. The scientists can control the pore size distribution of the gel substrate to enhance efficacy of the nanosensor. They tested the efficiency of the gel nanosensor to predict complex topographical dose profiles in anthromorphic head and neck phantoms and in live canine patients undergoing radiotherapy. The highly disruptive and translational potential of the gel nanosensor technology will lead to improved patient safety and outcomes in clinical radiotherapy.
© 2019 Science X Network
 Varme artikler
Varme artikler
-
 Kontrol af elektroner i grafen åbner en ny vej til potentielle elektroniske enhederStartende med et manglende atom, kaldet en ledig stilling (øverst til venstre), og anvende en elektrisk ladning, der tiltrækker elektroner til regionen, elektronerne er begrænset til orbitaler for at
Kontrol af elektroner i grafen åbner en ny vej til potentielle elektroniske enhederStartende med et manglende atom, kaldet en ledig stilling (øverst til venstre), og anvende en elektrisk ladning, der tiltrækker elektroner til regionen, elektronerne er begrænset til orbitaler for at -
 Ny undersøgelse afslører kommunikationspotentiale for grafenGrafene består af et enkelt lag carbonatomer. Kredit:Wikimedia Commons At levere sikre trådløse forbindelser og forbedre effektiviteten af kommunikationsenheder kan være en anden applikation til
Ny undersøgelse afslører kommunikationspotentiale for grafenGrafene består af et enkelt lag carbonatomer. Kredit:Wikimedia Commons At levere sikre trådløse forbindelser og forbedre effektiviteten af kommunikationsenheder kan være en anden applikation til -
 Forskere foreslår 3D grafen-lignende hyper-bikagestrukturerTo eksempler på 3D-gitter baseret på en kulstofbaseret plan trigonal struktur:(a) hyper-honningkagen og (b) en otte-atomet enhedscelle. En zoo af andre strukturer kan laves ved at skabe variationer af
Forskere foreslår 3D grafen-lignende hyper-bikagestrukturerTo eksempler på 3D-gitter baseret på en kulstofbaseret plan trigonal struktur:(a) hyper-honningkagen og (b) en otte-atomet enhedscelle. En zoo af andre strukturer kan laves ved at skabe variationer af -
 Injicerbare mikrosfærer til at reparere svigtende hjerterStamceller vokset over overfladen af mikrosfærerne. Kredit:University College London Bionedbrydelige mikrosfærer kan bruges til at levere hjerteceller genereret fra stamceller til at reparere be
Injicerbare mikrosfærer til at reparere svigtende hjerterStamceller vokset over overfladen af mikrosfærerne. Kredit:University College London Bionedbrydelige mikrosfærer kan bruges til at levere hjerteceller genereret fra stamceller til at reparere be
- Socialpolitikker kan ikke kun forbedre den økonomiske velfærd, men også sundhed
- Lovende ny legering til resistiv koblingshukommelse
- Yellowstone raslede af sværm af mere end 140 jordskælv i det sidste døgn, siger geologer
- E-handelsgiganten Alibaba hæver 11 milliarder dollars i aktienotering
- Påvirker klimaeffekterne af luftforurening den globale økonomi?
- Første resultater fra ICESat-2 missionskort 16 år med smeltende iskapper


