Do chemical reactions always make new substances?
Yes, chemical reactions *can* make new substances:
* Rearrangement of Atoms: Chemical reactions involve the breaking and forming of chemical bonds between atoms. This rearrangement of atoms leads to the creation of new molecules with different properties. For eksempel, når du brænder træ, kulstof, brint og iltatomer i træet omarrangeres for at danne kuldioxid, vand og aske - stoffer med helt forskellige egenskaber.
However, chemical reactions can also involve:
* Phase Changes: These are physical changes, not chemical changes. Melting ice (solid water) into liquid water or boiling water into steam (gaseous water) does not create new substances. The water molecules remain H₂O.
* Mixing: Mixing substances like sugar and water creates a solution, but the sugar and water molecules themselves remain unchanged.
To summarize:
* Chemical reactions *can* and often *do* form new substances by rearranging atoms.
* Some reactions involving phase changes or mixing do not form new substances.
So, it depends on the specific chemical reaction!
 Varme artikler
Varme artikler
-
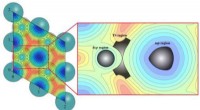 Nøjagtig og effektiv dynamisk beregningsstrategi for heterogen katalyseKredit:Prof. Zhuangs gruppe Teoretisk beregning er blevet en uundværlig tilgang til at afsløre termodynamikken og kinetikken i katalyse. Statisk beregningsstrategi er den mest populære tilgang i te
Nøjagtig og effektiv dynamisk beregningsstrategi for heterogen katalyseKredit:Prof. Zhuangs gruppe Teoretisk beregning er blevet en uundværlig tilgang til at afsløre termodynamikken og kinetikken i katalyse. Statisk beregningsstrategi er den mest populære tilgang i te -
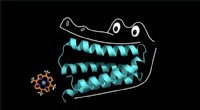 Labfremstillede proteinkugle co-faktor som en stor ol gatorDet syntetiske protein klemmer sig fast på porphyrinen som en alligators kæber. Kredit:Nicholas Polizzi Proteiner har magt til at turbolade biokemiske reaktioner inde i kroppen. Uden hjælp af pro
Labfremstillede proteinkugle co-faktor som en stor ol gatorDet syntetiske protein klemmer sig fast på porphyrinen som en alligators kæber. Kredit:Nicholas Polizzi Proteiner har magt til at turbolade biokemiske reaktioner inde i kroppen. Uden hjælp af pro -
 Nyheder om bæredygtig plante-afledt plast til at hjælpe organforskningDr. Maïwenn Kersaudy-Kerhoas og Alfredo Ongaro arbejder i laboratoriet med PLA. Kredit:Heriot-Watt University Dr. Maïwenn Kersaudy-Kerhoas og Alfredo Ongaro fra Institute of Biological Chemistry,
Nyheder om bæredygtig plante-afledt plast til at hjælpe organforskningDr. Maïwenn Kersaudy-Kerhoas og Alfredo Ongaro arbejder i laboratoriet med PLA. Kredit:Heriot-Watt University Dr. Maïwenn Kersaudy-Kerhoas og Alfredo Ongaro fra Institute of Biological Chemistry, -
 Nyt twist på CRISPR-teknologiUniversity of Delaware doktorand Emily Berckman (til venstre) og prof. Wilfred Chen har fundet en ny måde at bruge CRISPR -teknologi, der vil hjælpe kemikere, biokemikere og ingeniører, der arbejder m
Nyt twist på CRISPR-teknologiUniversity of Delaware doktorand Emily Berckman (til venstre) og prof. Wilfred Chen har fundet en ny måde at bruge CRISPR -teknologi, der vil hjælpe kemikere, biokemikere og ingeniører, der arbejder m
- Hvordan påvirker massen af et objekt resultatet, når ubalanceret kraft virker på det?
- Hvad er det voksagtige lag, der omgiver blad og proctekter det fra for meget vandtab kaldet?
- Hvorfor er Celsius en nyttig temperaturskala?
- Naturligt isolerende materiale udsender pulser af superfluorescerende lys ved stuetemperatur
- Forskere opnår høj effekt med ny mindre laser
- Ny undersøgelse:Fracking forårsager global stigning i atmosfærisk metan


