Who disproved the Rutherford model of atom?
Her er hvorfor:
* Rutherford's Model: Rutherford's model, proposed in 1911, was a revolutionary step forward. It established that atoms have a dense, positively charged nucleus with electrons orbiting around it. This was a significant departure from the earlier "plum pudding" model.
* Begrænsninger: The Rutherford model, however, had some limitations:
* It couldn't explain why electrons didn't spiral into the nucleus due to electromagnetic forces.
* It couldn't account for the line spectra observed in atomic emissions.
* Bohr Model: Niels Bohr's model, proposed in 1913, addressed these limitations by introducing the concept of quantized energy levels for electrons. Bohrs model oplyste, at elektroner kun kunne eksistere i specifikke kredsløb, hver med et fast energiniveau, og at de kunne hoppe mellem disse niveauer ved at absorbere eller udsende fotoner.
* Quantum Mechanics: The Bohr model was a significant improvement, but it too had its limitations. The development of quantum mechanics in the 1920s provided a much more comprehensive and accurate description of atomic structure and behavior.
Kortfattet:
* Rutherford's model laid the foundation for our understanding of atomic structure.
* Bohr's model refined it by incorporating quantization of energy levels.
* Quantum mechanics provided a more complete and accurate model.
Therefore, it's more accurate to say that later scientific discoveries built upon and expanded the Rutherford model rather than "disproving" it.
 Varme artikler
Varme artikler
-
 Hårde enkeltmolekylemagneter:Tetranukleære sjældne jordarters metalkomplekser med kæmpe spinKredit:Wiley Magneter dannet af et enkelt molekyle er af særlig interesse i datalagring, da evnen til at gemme lidt på hvert molekyle kunne øge computerens lagerkapacitet markant. Forskere har nu
Hårde enkeltmolekylemagneter:Tetranukleære sjældne jordarters metalkomplekser med kæmpe spinKredit:Wiley Magneter dannet af et enkelt molekyle er af særlig interesse i datalagring, da evnen til at gemme lidt på hvert molekyle kunne øge computerens lagerkapacitet markant. Forskere har nu -
 Hærens forskning lyser vejen for nye materialerUS Army Research Laboratory forskere Dr. David Baker og Dr. Joshua McClure poserer i deres laboratorium på Adelphi Laboratory Center, hvor de arbejder på at lette byrden og forbedre styrken af Soldi
Hærens forskning lyser vejen for nye materialerUS Army Research Laboratory forskere Dr. David Baker og Dr. Joshua McClure poserer i deres laboratorium på Adelphi Laboratory Center, hvor de arbejder på at lette byrden og forbedre styrken af Soldi -
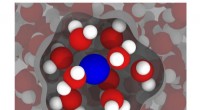 Moderne simuleringer kan forbedre MRI'erEn illustration baseret på simuleringer fra Rice Universitys ingeniører viser en gadoliniumion (blå) i vand (rød og hvid), med vand i den indre sfære - det vand, der er mest påvirket af gadolinium - f
Moderne simuleringer kan forbedre MRI'erEn illustration baseret på simuleringer fra Rice Universitys ingeniører viser en gadoliniumion (blå) i vand (rød og hvid), med vand i den indre sfære - det vand, der er mest påvirket af gadolinium - f -
 Sætte fejl på menuen, sikkertKredit:Pixabay/CC0 Public Domain Tanken om at spise insekter er mavedrejning for mange, men ny forskning fra Edith Cowan University (ECU) kaster lys over allergifremkaldende proteiner, som kan udg
Sætte fejl på menuen, sikkertKredit:Pixabay/CC0 Public Domain Tanken om at spise insekter er mavedrejning for mange, men ny forskning fra Edith Cowan University (ECU) kaster lys over allergifremkaldende proteiner, som kan udg
- Hvordan man laver en Ladybug Habitat
- Er Gabbro en påtrængende eller ekstruderende stødende rock, hvordan ved du det?
- Til månen og tilbage:NASAs Artemis II-besætning øver splashdown
- Sådan vælger du blandt præsidentkandidater, du ikke kan lide
- Hvad ville der ske, hvis mikroorganismerne blev fjernet fra fødekæden?
- Vulkanudbrud påvirkede Islands omvendelse til kristendommen


