What compound is Si3F3?
* Valency: Silicon (Si) has a valency of +4, meaning it can form four bonds. Fluorine (F) has a valency of -1, meaning it can form one bond.
* støkiometri: The formula Si3F3 suggests a 3:3 ratio of silicon to fluorine. However, this ratio wouldn't allow all the atoms to satisfy their valency requirements.
Possible Compounds:
* Silicon tetrafluoride (SiF4): This is a common compound with silicon having four fluorine atoms bonded to it (SiF4).
* Trisilicon octafluoride (Si3F8): This is a less common compound, but it would allow all atoms to satisfy their valencies.
Konklusion:
The compound Si3F3 is not a stable, neutral compound because it violates the valency rules of its constituent elements. The correct formulas for silicon-fluorine compounds are SiF4 and Si3F8.
Sidste artikelHvad er ladningen på kation i ionisk sammensat bariumsulfid?
Næste artikelEr ammoniumchloridopløselig i ethanol?
 Varme artikler
Varme artikler
-
 Ny indsigt i strukturen af et dræberproteinStephanie Bleicken (til venstre), Enrica Bordignon, og Tufa Assafa undersøgte proteinet ved hjælp af forskellige spektroskopiske teknikker. Kredit:RUB, Kramer Forskere ved Ruhr-Universität Bochum
Ny indsigt i strukturen af et dræberproteinStephanie Bleicken (til venstre), Enrica Bordignon, og Tufa Assafa undersøgte proteinet ved hjælp af forskellige spektroskopiske teknikker. Kredit:RUB, Kramer Forskere ved Ruhr-Universität Bochum -
 Molekylært stillads til modificering af fluorescerende forbindelser, der anvendes til biologisk bil…Et bibliotek af farvestofmolekyler syntetiseret med en modulær kemisk tilgang kan føre til forbedret billeddannelse af levende celler. Kredit:A*STAR Institute of Chemical and Engineering Sciences
Molekylært stillads til modificering af fluorescerende forbindelser, der anvendes til biologisk bil…Et bibliotek af farvestofmolekyler syntetiseret med en modulær kemisk tilgang kan føre til forbedret billeddannelse af levende celler. Kredit:A*STAR Institute of Chemical and Engineering Sciences -
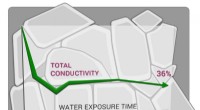 Forskere viser, at en lovende fast elektrolyt er hydrofobSkoltech-forskere og deres kolleger har vist, at LATP, en fast elektrolyt, der overvejes til brug i næste generations energilagring, er meget følsom over for vand, hvilket har direkte konsekvenser for
Forskere viser, at en lovende fast elektrolyt er hydrofobSkoltech-forskere og deres kolleger har vist, at LATP, en fast elektrolyt, der overvejes til brug i næste generations energilagring, er meget følsom over for vand, hvilket har direkte konsekvenser for -
 Forskere på forkant med udvikling af maskinlæringsmetoder til kemisk opdagelseKredit:CC0 Public Domain Opdagelsen og formuleringen af nye lægemidler, antivirale midler, antibiotika og generelt kemikalier med skræddersyede egenskaber er en lang og omhyggelig proces. Tværfa
Forskere på forkant med udvikling af maskinlæringsmetoder til kemisk opdagelseKredit:CC0 Public Domain Opdagelsen og formuleringen af nye lægemidler, antivirale midler, antibiotika og generelt kemikalier med skræddersyede egenskaber er en lang og omhyggelig proces. Tværfa


