Udviklingen af slim:Hvordan fik vi alt dette slim?

Petar Pajic, UB ph.d.-studerende i biologiske videnskaber, forbereder en spytprøve til separation og analyse. I den nye undersøgelse brugte holdet en gelelektroforeseteknik til at adskille muciner fra andre proteiner i spyt hos forskellige pattedyr. Kredit:Douglas Levere / University at Buffalo
Fra slimbelægningssnegle til spyt i vores mund indeholder mange glatte kropsvæsker slim. Så hvordan udviklede dette vidunder af biologi sig?
Hos pattedyr er svaret mange gange, og ofte på en overraskende måde, ifølge en ny undersøgelse af proteiner kaldet muciner. Disse molekyler har en række forskellige funktioner, men som en familie er de kendt som komponenter af slim, hvor de bidrager til stoffets klistrede konsistens.
Gennem en sammenligning af mucin-gener i 49 pattedyrarter identificerede forskere 15 tilfælde, hvor nye muciner ser ud til at have udviklet sig gennem en additiv proces, der transformerede et ikke-mucin-protein til et mucin.
Forskerne foreslår, at hver af disse "mucinisering"-begivenheder begyndte med et protein, der ikke var et mucin. På et tidspunkt satte evolutionen et nyt afsnit på denne ikke-mucinbase:en bestående af en kort kæde af byggesten kaldet aminosyrer, der er dekoreret med sukkermolekyler. Med tiden blev denne nye region duplikeret, med flere kopier tilføjet for at forlænge proteinet yderligere, hvilket gør det til et mucin.
De fordoblede regioner, kaldet "gentagelser", er nøglen til en mucins funktion, siger forskere fra University at Buffalo Omer Gokcumen og Stefan Ruhl, seniorforfatterne til undersøgelsen, og Petar Pajic, den første forfatter.
Sukkeret, der dækker disse sektioner, rager udad som børsterne på en flaskebørste, og de giver muciner den slimede egenskab, der er afgørende for mange vigtige opgaver, som disse proteiner udfører.
Forskningen vil blive offentliggjort den 26. august i Science Advances.
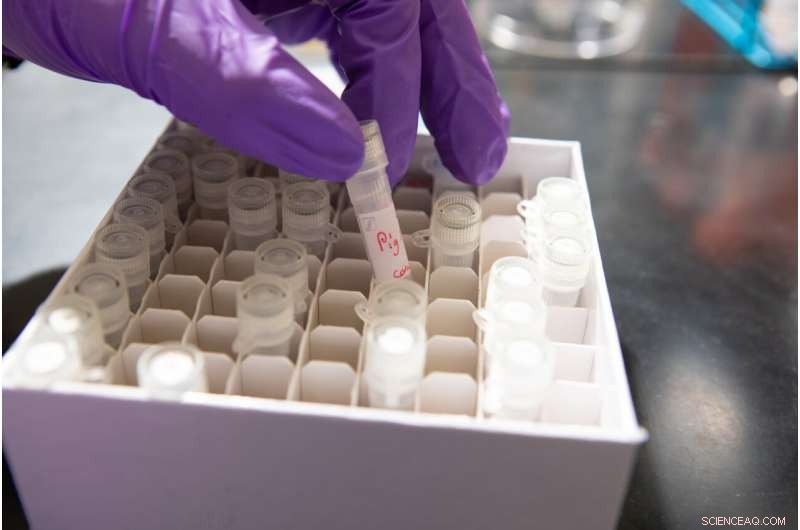
Hætteglas med spyt indsamlet fra forskellige pattedyr, herunder en gris. Kredit:Douglas Levere / University at Buffalo
"Jeg tror ikke, det tidligere var kendt, at proteinfunktion kan udvikle sig på denne måde, fra et protein får gentagne sekvenser. Et protein, der ikke er et mucin, bliver til et mucin bare ved at få gentagelser. Dette er en vigtig måde, hvorpå evolutionen laver slim Det er et evolutionært trick, og vi dokumenterer nu, at dette sker igen og igen," siger Gokcumen, Ph.D., lektor i biologiske videnskaber ved UB College of Arts and Sciences.
"De gentagelser, vi ser i muciner, kaldes 'PTS-gentagelser' for deres høje indhold af aminosyrerne prolin, threonin og serin, og de hjælper muciner i deres vigtige biologiske funktioner, der spænder fra at smøre og beskytte vævsoverflader til at hjælpe med at gøre vores mad glat så vi kan sluge det,« siger Stefan Ruhl, DDS, Ph.D., midlertidig dekan på UB School of Dental Medicine og professor i oral biologi. "Gavnlige mikrober har udviklet sig til at leve på slimbelagte overflader, mens slim på samme tid også kan fungere som en beskyttende barriere og forsvare mod sygdom ved at beskytte os mod uønskede patogene ubudne gæster."
"Ikke mange mennesker ved, at det første mucin, der var blevet renset og biokemisk karakteriseret, kom fra en spytkirtel," tilføjer Ruhl. "Mit laboratorium har studeret muciner i spyt i de sidste 30 år, mest fordi de beskytter tænderne mod forfald, og fordi de hjælper med at balancere mikrobiotaen i mundhulen."
Den spændende udvikling af et 'fantastisk livsegenskab'
"I think this paper is really interesting," Gokcumen says. "It's one of those times where we got lucky. We were studying saliva, and then we found something that's interesting and cool and decided to look into it."
While studying saliva, the team noticed that a small salivary mucin in humans called MUC7 was not present in mice. The rodents did, however, have a similarly sized salivary mucin called MUC10. The scientists wanted to know:Were these two proteins related from an evolutionary perspective?
The answer was no. But what the research uncovered next was a surprise. While MUC10 did not appear to be related to MUC7, a protein found in human tears called PROL1 did share a portion of MUC10's structure. PROL1 looked a lot like MUC10, minus the sugar-coated bottlebrush repeats that make MUC10 a mucin.
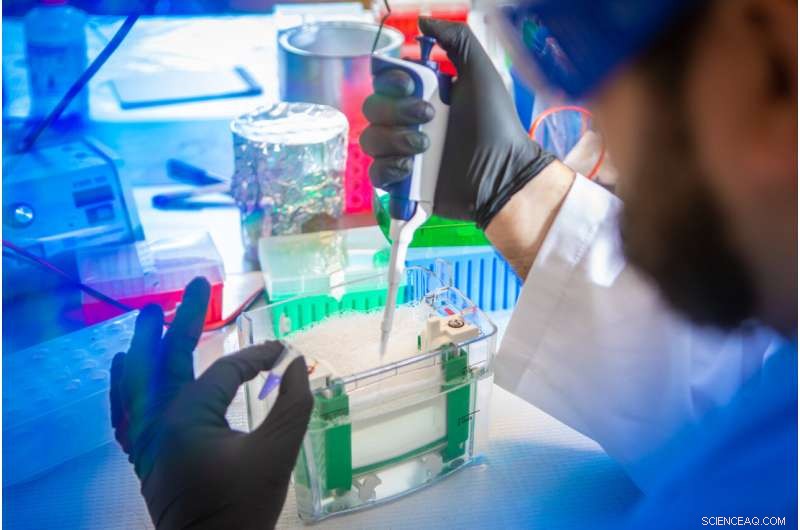
Petar Pajic, UB PhD student in biological sciences, uses a gel electrophoresis technique to separate mucins from other proteins in a saliva sample. Credit:Douglas Levere / University at Buffalo
"We think that somehow that tear gene ends up repurposed," Gokcumen says. "It gains the repeats that give it the mucin function, and it's now abundantly expressed in mouse and rat saliva."
The scientists wondered whether other mucins might have formed the same way. They began to investigate and discovered multiple examples of the same phenomena. Though many mucins share common ancestry among various groups of mammals, the team documented 15 instances in which evolution appeared to have converted non-mucin proteins into mucins via the addition of PTS repeats.
And this was "with a pretty conservative look," Gokcumen says, noting that the study focused on one region of the genome in a few dozen mammal species. He calls slime an "amazing life trait," and he's curious whether the same evolutionary mechanism might have driven the formation of some mucins in slugs, slime eels and other critters. More research is needed to find an answer.
"How new gene functions evolve is still a question we are asking today," says Pajic, a UB Ph.D. student in biological sciences. "Thus, we are adding to this discourse by providing evidence of a new mechanism, where gaining repeated sequences within a gene births a novel function."
"I think this could have even broader implications, both in understanding adaptive evolution and in possibly explaining certain disease-causing variants," Pajic adds. "If these mucins keep evolving from non-mucins over and over again in different species at different times, it suggests that there is some sort of adaptive pressure that makes it beneficial. And then, at the other end of the spectrum, maybe if this mechanism goes 'off the rails'—happening too much, or in the wrong tissue—then maybe it can lead to disease like certain cancers or mucosal illnesses."
The study on mucins demonstrates how a long-time partnership between evolutionary biologists and dental researchers at UB is yielding new insights into genes and proteins that are also important to human health.
"My team has been studying mucins for many decades, and my collaboration with Dr. Gokcumen has brought this research to a new level by revealing all these exciting novel insights into their evolutionary genetics," Ruhl says. "At this advanced stage of my career, it is also immensely gratifying to see that the flame of scientific curiosity is being carried on by a new generation of young investigators like Petar Pajic." + Udforsk yderligere
Contact lenses:A molecule from pig stomach mucus prevents corneal damage
 Varme artikler
Varme artikler
-
 Planter udvikler dufte og farver for at tiltrække dyr til spredning af frøPå Madagaskar, spiselige frugter, såsom figner, har udviklet sig til at være ekstremt duftende og for det meste gule, en farve mere synlig for lemurer, som er rødgrøn farveblind. Wikimedia Commons CC
Planter udvikler dufte og farver for at tiltrække dyr til spredning af frøPå Madagaskar, spiselige frugter, såsom figner, har udviklet sig til at være ekstremt duftende og for det meste gule, en farve mere synlig for lemurer, som er rødgrøn farveblind. Wikimedia Commons CC -
 Sporing af et plantetrin:Efter frøspredning ved hjælp af chloroplast -DNACaliforniens guldfelter ( Lasthenia californica ) vokser i det sydlige Oregon, USA. Kredit:Monica Grasty Planter spreder deres frø ud over landskabet for at kolonisere nye områder, men det er sv
Sporing af et plantetrin:Efter frøspredning ved hjælp af chloroplast -DNACaliforniens guldfelter ( Lasthenia californica ) vokser i det sydlige Oregon, USA. Kredit:Monica Grasty Planter spreder deres frø ud over landskabet for at kolonisere nye områder, men det er sv -
 Ja,Neanderthalere kunne grine Neanderthaleren kunne helt sikkert have grinet, men hvad ville have kildret den neandertaleriske sjove knogle? Det ved vi nok aldrig. Flickr I årtusinder, mennesker
Ja,Neanderthalere kunne grine Neanderthaleren kunne helt sikkert have grinet, men hvad ville have kildret den neandertaleriske sjove knogle? Det ved vi nok aldrig. Flickr I årtusinder, mennesker -
 Hvor finder respiration sted?Den kemiske reaktion kaldet respiration er afgørende for vækst, reparation og overlevelse af alle levende ting. Respiration sker i cellerne i planter, dyr og mennesker, hovedsageligt inden i mitokondr
Hvor finder respiration sted?Den kemiske reaktion kaldet respiration er afgørende for vækst, reparation og overlevelse af alle levende ting. Respiration sker i cellerne i planter, dyr og mennesker, hovedsageligt inden i mitokondr
- Hård vinterstorm i USA set aftagende
- I den nedværdigende natur skader menneskeheden sig selv, FN-rapport advarer
- Forskere udvikler metoder til at opbygge funktionelle elementer i kvantecomputere
- Hvad er forskellen mellem konstruktive og destruktive jordprocesser?
- Forskere beregner jordens frysedybde ud fra satellitdata
- Ekstreme naturbrande gør røg-apps til de nye vejr-apps


