Hvordan forskere opdagede en ny måde at producere actinium-225, en sjælden medicinsk radioisotop
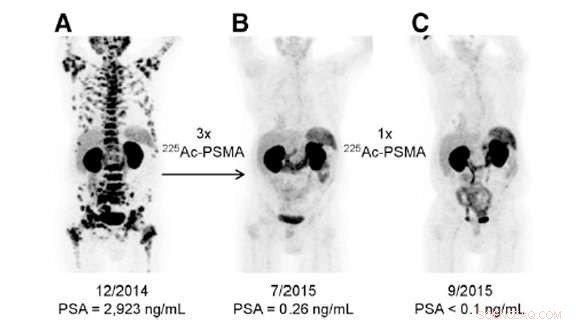
Dette billede viser tre forskellige billeder af en enkelt patient med prostatakræft i slutstadiet. Den første blev taget før behandling med actinium-225, den anden efter tre doser, og den tredje efter en ekstra dosis. Behandlingen, udført på Universitetshospitalet Heidelberg, var yderst vellykket. Kredit:US Department of Energy
Inde i et smalt glasrør sidder et stof, der kan skade eller helbrede, afhængigt af hvordan du bruger det. Det afgiver en svag blå glød, et tegn på dets radioaktivitet. Mens energien og de subatomære partikler, den udsender, kan beskadige menneskelige celler, de kan også dræbe nogle af vores mest genstridige kræftformer. Dette stof er actinium-225.
Heldigvis, forskere har fundet ud af, hvordan man udnytter actinium-225's magt for godt. De kan vedhæfte det til molekyler, der kun kan bruges på kræftceller. I kliniske forsøg med behandling af prostatakræftpatienter i sen fase, actinium-225 udslettede kræften i tre behandlinger.
"Der er ingen restvirkninger af prostatakræft. Det er bemærkelsesværdigt, "sagde Kevin John, en forsker ved Department of Energy's (DOE) Los Alamos National Laboratory (LANL). Actinium-225 og behandlinger afledt af det er også blevet brugt i tidlige forsøg med leukæmi, melanom, og gliom.
Men noget stod i vejen for at udvide denne behandling.
I årtier, ét sted i verden har produceret størstedelen af actinium-225:DOE's Oak Ridge National Laboratory (ORNL). Selv med to andre internationale faciliteter, der bidrager med mindre beløb, alle tre tilsammen kan kun skabe nok aktinium-225 til at behandle færre end 100 patienter årligt. Det er ikke nok til at køre andet end de mest foreløbige af kliniske forsøg.
For at opfylde sin mission om at producere isotoper, der er mangelvare, DOE Office of Science's Isotope Program leder bestræbelser på at finde nye måder at producere actinium-225 på. Gennem DOE Isotope-programmets Tri-Lab Research-indsats for at levere acceleratorproduceret 225Ac til strålebehandling-projekt, ORNL, LANL, og DOE's Brookhaven National Laboratory (BNL) har udviklet en ny, ekstremt lovende proces til fremstilling af denne isotop.
Bygger på en arv fra atomalderen
At producere isotoper til medicinsk og anden forskning er ikke noget nyt for DOE. Isotop -programmets oprindelse stammer fra 1946, som en del af præsident Trumans bestræbelser på at udvikle fredelige anvendelser af atomenergi. Siden da, Atomenergikommissionen (DOE's forgænger) og DOE har fremstillet isotoper til forskning og industriel anvendelse. De unikke udfordringer, der følger med isotopproduktion, gør DOE velegnet til denne opgave.
Isotoper er forskellige former for standardatomiske elementer. Mens alle former for et element har samme antal protoner, isotoper varierer i deres antal neutroner. Nogle isotoper er stabile, men de fleste er ikke. Ustabile isotoper forfalder konstant, udsender subatomære partikler som radioaktivitet. Når de frigiver partikler, isotoper ændres til forskellige isotoper eller endda forskellige elementer. Kompleksiteten ved at producere og håndtere disse radioaktive isotoper kræver ekspertise og specialudstyr.
DOE -isotopprogrammet fokuserer på fremstilling og distribution af isotoper, der er mangelvare og stor efterspørgsel, vedligeholde infrastrukturen til at gøre det, og udfører forskning for at producere isotoper. Det fremstiller isotoper, som private virksomheder ikke gør kommercielt tilgængelige.
En enestående kræftkæmper
Produktion af actinium-225 bringer de nationale laboratoriers ekspertise ind i et nyt område.
Actinium-225 har et sådant løfte, fordi det er en alfa-emitter. Alfa -emittere afgiver alfapartikler, som er to protoner og to neutroner bundet sammen. Når alfapartikler forlader et atom, de deponerer energi langs deres korte vej. Denne energi er så høj, at den kan bryde bindinger i DNA. Denne skade kan ødelægge kræftcellernes evne til at reparere og formere sig, endda dræbe tumorer.
"Alpha -emittere kan fungere i tilfælde, hvor intet andet virker, "sagde Ekaterina (Kate) Dadachova, en forsker ved University of Saskatchewan College of Pharmacy and Nutrition, der testede actinium-225 produceret af DOE.
Imidlertid, uden en måde at målrette kræftceller på, alfa -emittere ville være lige så skadelige for raske celler. Forskere knytter alfa -emittere til et protein eller antistof, der præcist matcher receptorerne på kræftceller, som at montere en lås i en nøgle. Som resultat, alfa -emitteren akkumuleres kun på kræftcellerne, hvor den udsender sine destruktive partikler over en meget kort afstand.
"Hvis molekylet er designet korrekt og går til selve målet, du dræber kun de celler, der er omkring den målrettede celle. You do not kill the cells that are healthy, " said Saed Mirzadeh, an ORNL researcher who began the initial effort to produce actinium-225 at ORNL.
Actinium-225 is unique among alpha emitters because it only has a 10-day half-life. (An isotope's half-life is the amount of time it takes to decay to half of its original amount.) In fewer than two weeks, half of its atoms have turned into different isotopes. Neither too long nor too short, 10 days is just right for some cancer treatments. The relatively short half-life limits how much it accumulates in people's bodies. På samme tid, it gives doctors enough time to prepare, administer, and wait for the drug to reach the cancer cells in patients' bodies before it acts.
Repurposing Isotopes for Medicine
While it took decades for medical researchers to figure out the chemistry of targeting cancer with actinium-225, the supply itself now holds research back. I 2013, the federal Food and Drug Administration (FDA) approved the first drug based on alpha emitters. If the FDA approves multiple drugs based on actinium-225 and its daughter isotope, bismuth-213, demand for actinium-225 could rise to more than 50, 000 millicuries (mCi, a unit of measurement for radioactive isotopes) a year. The current process can only create two to four percent of that amount annually.
"Having a short supply means that much less science gets done, " said David Scheinberg, a Sloan Kettering Institute researcher who is also an inventor of technology related to the use of actinium-225. (This technology has been licensed by the Sloan Kettering Institute at the Memorial Sloan Kettering Cancer Center to Actinium Pharmaceuticals, for which Scheinberg is a consultant.)
Part of this scarcity is because actinium is remarkably rare. Actinium-225 does not occur naturally at all.
Scientists only know about actinium-225's exceptional properties because of a quirk of history. I 1960'erne, scientists at the DOE's Hanford Site produced uranium-233 as a fuel for nuclear weapons and reactors. They shipped some of the uranium-233 production targets to ORNL for processing. Those targets also contained thorium-229, which decays into actinium-225. I 1994, a team from ORNL led by Mirzadeh started extracting thorium-229 from the target material. They eventually established a thorium "cow, " from which they could regularly "milk" actinium-225. In August 1997, they made their first shipment of actinium-225 to the National Cancer Institute.
I øjeblikket, scientists at ORNL "milk" the thorium-229 cow six to eight times a year. They use a technique that separates out ions based on their charges. Desværre, the small amount of thorium-229 limits how much actinium-225 scientists can produce.
Accelerating Actinium-225 Research
Ultimativt, the Tri-Lab project team needed to look beyond ORNL's radioactive cow to produce more of this luminous substance.
"The route that looked the most promising was using high-energy accelerators to irradiate natural thorium, " said Cathy Cutler, the director of BNL's medical isotope research and production program.
Only a few accelerators in the country create high enough energy proton beams to generate actinium-225. BNL's Linear Accelerator and LANL's Neutron Science Center are two of them. While both mainly focus on other nuclear research, they create plenty of excess protons for producing isotopes.
The new actinium-225 production process starts with a target made of thorium that's the size of a hockey puck. Scientists place the target in the path of their beam, which shoots protons at about 40 percent the speed of light. As the protons from the beam hit thorium nuclei, they raise the energy of the protons and neutrons in the nuclei. The protons and neutrons that gain enough kinetic energy escape the thorium atom. Ud over, some of the excited nuclei split in half. The process of expelling protons and neutrons as well as splitting transforms the thorium atoms into hundreds of different isotopes – of which actinium-225 is one.
After 10 days of proton bombardment, scientists remove the target. They let the target rest so that the short-lived radioisotopes can decay, reducing radioactivity. They then remove it from its initial packaging, analyze it, and repackage it for shipping.
Then it's off to ORNL. Scientists there receive the targets in special containers and transfer them to a "hot cell" that allows them to work with highly radioactive materials. They separate actinium-225 from the other materials using a similar technique to the one they use to produce "milk" from their thorium cow. They determine which isotopes are in the final product by measuring the isotopes' radioactivity and masses.
Trials and Tribulations
Figuring out this new process was far from easy.
Først, the team had to ensure the target would hold up under the barrage of protons. The beams are so strong they can melt thorium – which has a melting point above 3, 000 degrees F. Scientists also wanted to make it as easy as possible to separate the actinium-225 from the target later on.
"There's a lot of work that goes into designing that target. It's really not a simple task at all, " said Cutler.
Næste, the Tri-Lab team needed to set the beamlines to the right parameters. The amount of energy in the beam determines which isotopes it produces. By modeling the process and then conducting trial-and-error tests, they determined settings that would produce as much actinium-225 as possible.
But only time and testing could resolve the biggest challenge. While sorting actinium out from the soup of other isotopes was difficult, the ORNL team could do it using fairly standard chemical practices. What they can't do is separate out the actinium-225 from its longer-lived counterpart actinium-227. When the team ships the final product to customers, it has about 0.3 percent actinium-227. With a half-life of years rather than days, it could potentially remain in patients' bodies and cause damage for far longer than actinium-225 does.
To understand the consequences of the actinium-227 contamination, the Tri-Lab team collaborated with medical researchers, including Dadachova, to test the final product. After analyzing the material for purity and testing it on mice, the researchers found no significant differences between the actinium-225 produced using the ORNL and the accelerator method. The amount of actinium-227 was so miniscule that it "doesn't make any difference, " said Dadachova.
Happily Ever After?
Having resolved many of the biggest issues, the Tri-Lab project team is in the midst of working out the new process's details. They estimate they can provide more than 20 times as much actinium-225 to medical researchers as they were able to originally. Those researchers are now investigating what dosages would maximize effectiveness while minimizing the drug's toxicity. På samme tid, the national labs are pursuing upgrades to expand production to the level needed for a commercial drug. They're also working to make the entire process more efficient.
"Having a larger supply from the DOE is essential to expanding the trials to more and more centers, " said Scheinberg. With the Tri-Lab project ahead of schedule, it appears that the new production process for actinium-225 could lead to a better ending for more patients than ever before.
 Varme artikler
Varme artikler
-
 Smart materiale muliggør nye applikationer inden for autonom kørsel og robotikSmart materiale muliggør nye applikationer inden for autonom kørsel, robotik, og sensorteknologi. Kredit:University of Luxembourg Forskning ledet af forskere fra University of Luxembourg har vist
Smart materiale muliggør nye applikationer inden for autonom kørsel og robotikSmart materiale muliggør nye applikationer inden for autonom kørsel, robotik, og sensorteknologi. Kredit:University of Luxembourg Forskning ledet af forskere fra University of Luxembourg har vist -
 Detaljeret fotodissociationsdynamik af hydrogensulfid afsløretRotations- og nuklear-spin-niveauafhængig multi-kanal produktmåling og fotodissociationsmekanismer af H 2 S. Kredit:ZHAO Yarui og YUAN Kaijun Svovlbrinte (H 2 S) er et af de vigtigste molekyle
Detaljeret fotodissociationsdynamik af hydrogensulfid afsløretRotations- og nuklear-spin-niveauafhængig multi-kanal produktmåling og fotodissociationsmekanismer af H 2 S. Kredit:ZHAO Yarui og YUAN Kaijun Svovlbrinte (H 2 S) er et af de vigtigste molekyle -
 Hvad sker der med en eksoterm reaktion, hvis temperaturen hæves?Nogle kemiske reaktioner - som at brænde træ eller eksplodere TNT - frigiver varme til deres omgivelser. Kemikere kalder disse eksoterme reaktioner. Forøgelse af temperaturen påvirker en eksoterm r
Hvad sker der med en eksoterm reaktion, hvis temperaturen hæves?Nogle kemiske reaktioner - som at brænde træ eller eksplodere TNT - frigiver varme til deres omgivelser. Kemikere kalder disse eksoterme reaktioner. Forøgelse af temperaturen påvirker en eksoterm r -
 N-heterocykliske fosfiner:lovende katalysatorer til transferhydrogeneringRapporterede fosforarter med hydridisk reaktivitet. Kredit:Science China Press Hydridoverførsel er en fremherskende protokol til reduktion af umættede forbindelser, som traditionelt opnås under ka
N-heterocykliske fosfiner:lovende katalysatorer til transferhydrogeneringRapporterede fosforarter med hydridisk reaktivitet. Kredit:Science China Press Hydridoverførsel er en fremherskende protokol til reduktion af umættede forbindelser, som traditionelt opnås under ka
- Sådan beregnes prævalenssatser pr. Tusind
- Kvantificering af enkeltmolekyleoverflade forbedrede Raman-spredning med DNA origami-metamolekyler
- Er Yeti bare en flok bjørne? Genetik siger ja
- Bioinformatik-computerprogrammer hjælper biologer med at forstå iboende forstyrrede proteiner
- Undersøgelse puster nyt liv i 2,3 milliarder år gamle Great Oxidation Event
- Hold dine venner tæt på


